Cancer is a complex disease, presents as an altered expression, closely associated with abnormal growth leading to interference in the normal functioning of cells. The disruption of which is caused by effects of genotoxic agents from the environment or chemical or radiation carcinogens resulting in genomic instability at an early stage of cancer, which often revolves as leukoplakia, erythroplakia, oral submucous fibrosis and lichen planus [1].
Of the total 90-95% of the malignancy arising from oral cavity are oral squamous cell carcinoma, some of them are preceded by a certain lesions grouped under potentially malignant disorders. These groups of lesions pose a close affiliation with the usage of tobacco and cigarette smoking [2]. Intermittent thorough follow up of these potentially malignant disorders aims at prevention and detection at the earliest, thereby decreasing the incidence and shoot up the survival rate.
Premalignant nature of a lesion or condition will not be ascertained by means of clinical means alone, as not all those lesions that appear white and red end up malignant. Confirmatory histopathology is a definite requisite for any kind of mucosal lesion that exceeds two weeks period even after the causative irritants are removed [3]. Individuals diagnosed to have potentially malignant disorders, should be screened for the signs of malignant transformation. In the present study, high risk individuals with lichen planus were identified on the basis of presence of chromosomal aberrations in the form of micronucleated cells.
Micronuclei (Mn) are nothing but structures that get separated from the main nucleus during nuclear division. They can be either chromosome fragments or whole chromosome itself that lag behind at the anaphase. Micronuclei index (Mni) is considered as standard cytogenetic end point that is commonly applied in genetic toxicology. Mni can be assessed in cells like lymphocytes, erythrocytes and also exfoliated epithelial cells, that provide the quantitative genomic damage that has occurred in vivo. Exfoliated cells derived from rapidly dividing cells are the target cells of Mn. The advantage of Mn assay over cytogenetic assay is that it is a simple, least invasive technique that doesn’t require ex vivo nuclear division and is less time consuming [4].
Materials and Methods
The present cross-sectional study was conducted in Rajah Muthaiah Dental College and Hospital, Annamalai University, Chidambaram, Tamilnadu. The study involved 32 participants selected using coin flip method, who were further categorized into 22 individuals confirmed to have lichen planus lesions and 10 healthy disease free individuals. The study sample encompassed buccal smears from both the groups.
The exfoliating epithelial cells were scraped from the lesion area of OLP cases [Table/Fig-1] and normal buccal mucosa in the second group [Table/Fig-2]. The samples were collected with the aid of moist sterilized wooden spatula. The obtained samples were then transferred onto a clean glass slide containing 0.09M NaCl. The sample is them made into a thin smear. The obtained smears were air dried and fixed with 95% isopropyl alcohol and stained in Feulgen Rossenback solution. To provide a better contrast and avoid eye strain 1% fast green was used as the counter stain. The stained smears after complete drying were evaluated microscopically under x1000 oil immersion and Mn were scored per 500 epithelial cells in each sample [Table/Fig-3] Counting of the epithelial cells was done in a sequential order as illustrated in [Table/Fig-4].
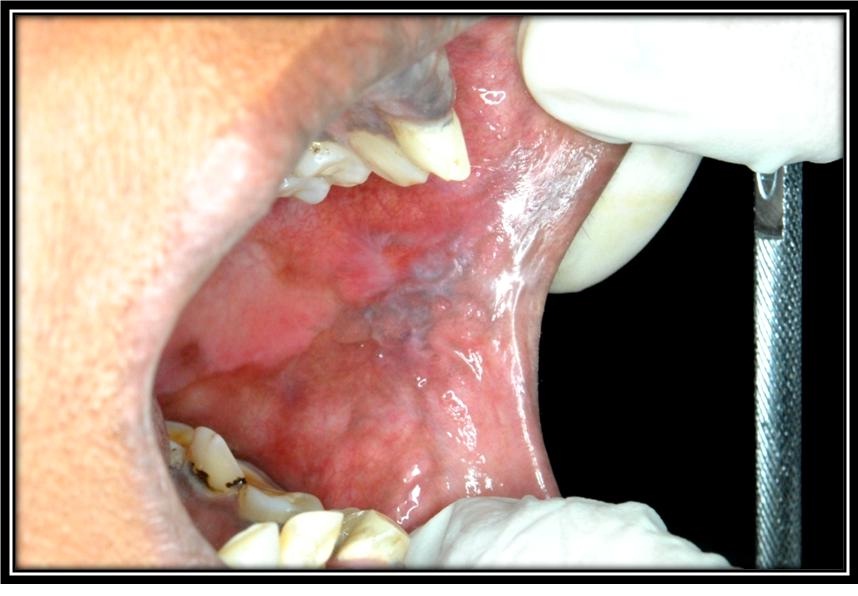
Patient with the lesional area of oral lichen planus in the buccal mucosa.
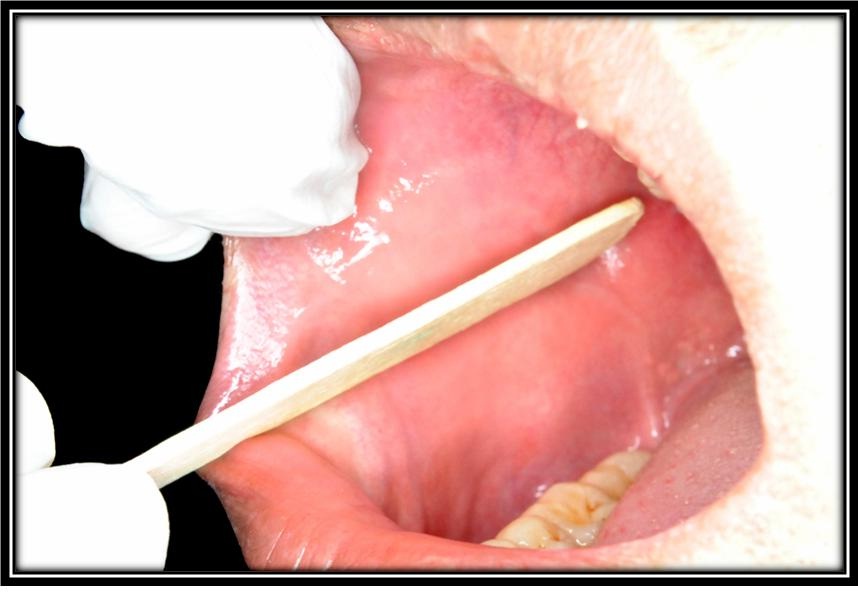
The epithelial cells were scraped from normal buccal mucosa using wooden spatula.
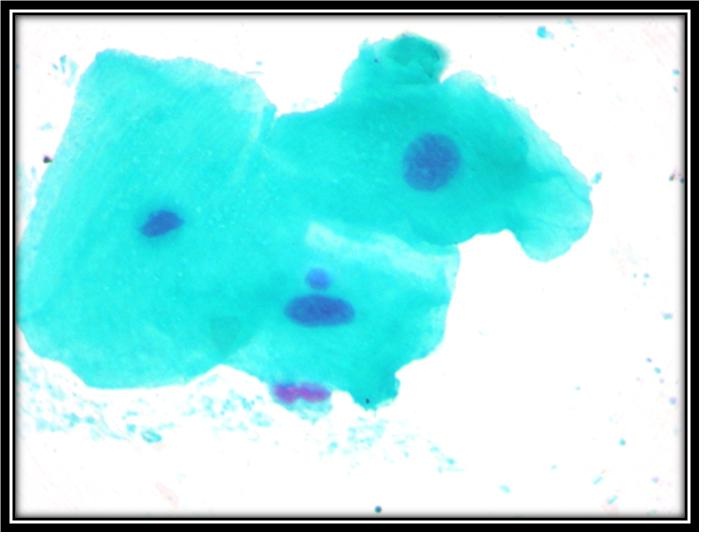
High power view of epithelial cells showing micronuclei.
Depicting counting of Mn in microscopic slide.
Mn are positive markers of accumulating genotoxicity. In the present study two different parameters were considered. The mean frequency of total number of Mn per 500 cells is calculated. The mean frequency of micronucleated cells (bearing at least one micronucleus) per 500 cells is also derived. Both the parameters are evaluated and statistically analysed. For Mn counting the criteria given by Tolbert et al., was followed [5].
Results
Total 22 samples (buccal smears) of clinically diagnosed cases of OLP and 10 samples of normal individuals were considered in the study. The smears of both the study and the control groups were stained in modified Feulgen-Rossenback reaction for Mn assay. Cytological evaluation of number of micronucleated cells (CMN), total number of micronuclei in micronucleated cells (TMN) was done for both OLP and control groups.
Number of micronucleated cells in 500 cells (CMN): The number of micronucleated cells per 500 cells (CMN) was evaluated in 22 samples of OLP cases and 10 samples of normal individuals. When CMN was compared among the study and control group, a mean value of 4.27 ± 1.80 and 0.90 ± 0.88 were obtained respectively. The results revealed a significant difference between OLP cases and normal individuals with respect to CMN (p<0.001) [Table/Fig-5].
Comparison of number of micro nucleated cells/500 cells (CMN) in OLP and control group using Kruskal Wallis test.
| CMN | N | Mean | SD | t- value | p-value |
|---|
| Study | 22 | 4.27 | 1.80 | 5.583 | 0.001 |
| Control | 10 | 0.90 | 0.88 |
Total number of micronuclei (TMN) in micronucleated cells: Total number of Mn in the micronucleated cells (TMN) was evaluated in samples of 22 OLP cases and 10 normal individuals. On comparing the TMN between the study and control groups, a mean value of 5.38 ± 2.42 and 1.5 ± 0.88 were obtained respectively. The results revealed a significant increase of TMN in the study than the control group (p<0.001) [Table/Fig-6].
Comparison of total number of micronuclei (TMN) in micronucleated cells between OLP and control group using Kruskal Wallis test.
| TMN | N | Mean | SD | t- value | p-value |
|---|
| Study | 22 | 5.38 | 2.42 | 5.224 | 0.001 |
| Control | 10 | 1.5 | 0.88 |
Frequency of number of micronucleated cells among the clinical variants of OLP cases: The frequency of micronucleated cells among the clinical variants {atrophic, reticular, erosive and combined (atrophic /erosive)} of OLP cases were observed and compared. Mean values of 4.50±0.55 (atrophic), 3.86±2.04 (reticular), 4.00±2.61 (erosive) and 6.00±0 (combined) were obtained in all the four groups of OLP samples. There was no statistically significant difference among the clinical variants of OLP with respect to frequency of CMN (p>0.05) [Table/Fig-7].
Comparison of CMN among the clinical variants of OLP samples using One way Anova test.
| CMN Vs Type of lesion | N | Mean | SD | f- value | p-value |
|---|
| Atrophic | 6 | 4.50 | 0.55 | 1.067 | 0.388(Not significant |
| Reticular | 7 | 3.86 | 2.04 |
| Erosive | 6 | 4.00 | 2.61 |
| Atrophic/Erosive | 3 | 6.00 | 0.00 |
| Total | 22 | 4.36 | 1.84 |
Frequency of total number of Mn among the clinical variants of OLP cases: The frequency of total number of micronuclei among the clinical variants, atrophic, reticular, erosive and combined (atrophic /erosive) of OLP cases were observed and compared. Mean values of 5.83±1.83 (atrophic), 4.00 ± 2.0(reticular), 4.83 ± 3.37(erosive) and 7.0±1.0 (combined) were obtained in all the four groups of OLP samples. There was no statistically significant difference among the clinical variants of OLP with respect to frequency of TMN, (p>0.5) [Table/Fig-8].
Comparison of TMN among the clinical variants of OLP using One way Anova test.
| CMN Vs Type of lesion | N | Mean | SD | f- value | p-value |
|---|
| Atrophic | 6 | 5.83 | 1.83 | 1.380 | 0.281(Not significant |
| Reticular | 7 | 4.00 | 2.00 |
| Erosive | 6 | 4.83 | 3.37 |
| Atrophic/Erosive | 3 | 7.00 | 1.00 |
| Total | 22 | 5.14 | 2.42 |
Discussion
OLP is a relatively common mucocutaneous disease with an incidence rate that equals cutaneous diseases like alopecia areata and psoriasis. The oral lesions of OLP have unique clinical, morphological features and an array of patterns described in literature. The clinical patterns of OLP include reticular, plaques, papules, atrophic, erosive ulcerative and bullous type [6–8].
There is profound documentation endorsing the role of cell mediated immunity, got triggered upon by the exogenous and endogenous factors in individuals who hold a genetic predisposition in evolving the disease [9–11]. The erosive clinical pattern of OLP is the predominant type and shows malignant transformation in future. The malignant transformation of OLP is an ongoing debate, whether the lesion has inherent potential, or OLP stands as a factor that might expedite the affectivity of carcinogen, or the presence of both OLP and squamous cell carcinoma could have been a bare synchrony [12].
The malignant transformation rate of OLP is between 0% and 5.8% [13]. Oral lesions of lichen planus clinically presents itself multifocally, simulating the process of field cancerization in oral cancer and high risk malignancies. Such high risk profile cases have to undergo a routine surveillance in order to spot the tumors in their earliest microinvasive phase. The aberrations at the cellular level need to be screened in intervals. The chromosomal aberrations if they get accumulated, they are observed as increased percentage of micronucleated cells and Mn in the buccal epithelial cells, a component of innate immunity [14].
The buccal micronucleus cytome assay (BMN assay) provides a platform to identify the high risk individuals by evaluating the markers of nuclear damage. Mn are extracytoplasmic nuclear bodies that result from loss of whole chromosomes or a portion of chromosome from the parent nuclei to the daughter nuclei during cell division. In the present study, micronucleus assay is cytogenetic assay that favors in identifying the DNA damage at single cell level [15].
The present study aimed to evaluate DNA damage in exfoliated buccal mucosal cells in individuals with OLP lesions and thereby to delineate the high risk group. Frequency of micronucleated exfoliated cells and the number of Mn in the exfoliated cells was compared between the study and the control group.
The percentage of number of Mn was 1.5% in the control and 5.38% in the study group. The percentage of micronucleated cells were 0.9% in the control and 4.27% in the study group. In our study, we included reticular, plaque, erosive and ulcerative OLP. Though the numbers were minimal a notable difference was observed among the variants, with the erosive and ulcerative patterns prevailing. Our study concurred that there is a remarkable increase noticed in the number of Mn and the micronucleated cells amidst the control and the study group.
Casartelli et al., in their study on comparing MN frequencies in the buccal cells of normal, precancerous and oral squamous cell carcinoma cases, concluded that there is a link of this biomarker with neoplastic progression, which is coincidence with our study [16]. Desai et al., also reported similar results in a study conducted on the exfoliated cells of patients with precancerous oral lesions [17]. Findings of the present study are also in accordance with those of Buajeeb et al., and Saran et al., [18,19]. The current study also validated the findings of Sanchez Siles et al., where on comparing the Mn frequency in the OLP and control groups, the OLP group showed a significant elevated Mn reflecting an ongoing DNA damage [15].
An inter-lesional comparison was made among the clinical variants to study the frequency of Mn and micronucleated cells within the study group. The highest frequency was found in combined lesions, followed by atrophic, erosive and finally the reticular variant of OLP. However, the results of the study were not statistically significant. This finding was also congruent with the findings of Buajeeb et al., [18]. These findings support the notion that there is an increased DNA damage in the study group.
To conclude, the findings from our study are strongly suggestive of an increased amount of DNA damage in OLP cases. Consequently, these OLP cases inevitably transform into oral squamous cell carcinomas more often than not.
Amongst the variants of OLP, erosive form is classified under premalignant lesions by WHO. One of the hidden agenda of our study was to witness the transformation of erosive OLP to malignancy, which is conceivable through a relentless follow-up for longer duration. Our study cases were followed for a period of six months. There was no frank malignant transformation observed during the follow-up period.
Conclusion
The present study clearly elicits that there is significant increase in the frequency of micronuclei and the micronucleated cells in the oral lichen planus as compared to normal individuals. Micronuclei reflect the transient DNA damage that has occurred in the tissue, the accumulation of which would be detrimental. Therefore, there is a greater risk of oral lichen planus lesions developing into oral squamous cell carcinoma.
The evaluation of frequency of micronuclei, although not pathognomic is instrumental in screening high risk population and subjects under carcinogenic risks. The results of the study also facilitates the application of micronuclei assay as a simple, fast chair side non invasive biomarker of cytogenetic damage.