Introduction
Hypothyroidism is one of the most common metabolic disorders associated with dyslipidemia which poses a higher risk of Coronary Artery Disease (CAD) in such patients. Biochemical markers which can pick up the risk promptly are becoming imperative now-a-days and thus the assessment beyond the conventional lipid profile is the need of the hour.
Aims
To assess the association of non-conventional lipid parameters like small dense Low Density Lipoprotein (sd LDL), oxidized Low Density Lipoprotein (ox LDL), Apolipoprotein A (Apo A1), Apolipoprotein B (Apo B) and Lipoprotein (a) {Lp(a)} in hypothyroid patients and compare their values with the conventional lipid parameters such as Total Cholesterol (TC), Triglyceride (TG), Low-Density Lipoprotein Cholesterol (LDL-C) and High-Density Lipoprotein Cholesterol (HDL-C).
Materials and Methods
One hundred and thirty clinically proven patients of hypothyroidism aged 20-60 years and equal number of age and gender matched healthy individuals were included in this case control study. Serum sd LDL, ox LDL, Apo A1, Apo B, Lp (a), lipid profile, Thyroid Stimulating Hormone (TSH), Free Triiodothyronine (FT3) and Free Tetraiodothyronine (FT4) levels were measured in both the groups. The data was recorded and analysed on SPSS system. The results of cases and controls were compared by student t-test and one-way ANOVA. All the parameters were correlated with TSH by Pearson’s correlation.
Results
We found significantly high levels of sd LDL, ox LDL, Apo B, Lp (a), TC, TG, LDL-C in cases as compared to the controls. Ox LDL has shown maximum correlation with serum TSH (p<0.0001, r=0.801) followed by sd LDL (p<0.0001, r=0.792), Apo B (p<0.001, r=0.783) and LDL-C (p<0.001, r=0.741). Moreover, ox LDL and sd LDL were found to be increased in normolipidemic hypothyroid patients thereby giving a strong supportive evidence that estimation of these parameters can become fundamental in prompt identification of the high risk patients of CAD in hypothyroid population.
Conclusion
Non-conventional lipid parameters appear to be better markers for the assessment of cardiovascular risk in hypothyroidism and might help in the designing of the effective treatment protocols and areas of intervention by the clinicians as well as researchers.
Introduction
Hypothyroidism is one of the most common endocrinal disorders, resulting from reduced activity of thyroid glands due to varied aetiology [1]. Primary hypothyroidism is due to the pathologies directly involving the functioning of thyroid glands while in secondary hypothyroidism, the thyroid glands are secondarily affected because of decreased synthesis of Thyroid Stimulating Hormone (TSH) in the anterior lobe of pituitary gland. Central causes of hypothyroidism typically present with other manifestations of hypothalamic or pituitary dysfunction and are characterized by inappropriately normal or low levels of TSH relative to the insufficient thyroid hormone. On the other hand, primary thyroid gland failure may occur due to congenital abnormalities, autoimmune destruction, iodine deficiency or infiltrative diseases. In such cases, decreased levels of tetraiodothyronine (T4) and tri-iodothyronine (T3) result in feedback stimulation of pituitary glands which results in increased synthesis of TSH which tries to increase the secretion of T4 and T3 [2]. In subclinical hypothyroidism, the increased level of TSH is able to maintain the thyroid hormones in normal range but in clinical or overt hypothyroidism even the increased levels of TSH are not able to maintain thyroid hormones in the normal range which leads to clinical manifestation. Therefore, increased level of TSH is one of the most sensitive laboratory findings in primary hypothyroidism [3].
About 300 million patients are suffering from thyroid disorders all over the world. About 42 million patients reside in India. The prevalence of hypothyroidism in India is 4-15 % as compared to the western countries where prevalence is approximately 4%. The risk of hypothyroidism in females (15.8%) is three times more as compared to the males (5.01%) [4].
Thyroid hormones serve wide array of functions in the body involving almost all the organs. At the molecular level, they are involved in the regulation of various metabolic pathways pertaining to carbohydrates, lipids, proteins, minerals and electrolytes. Thyroid hormones play an important role in the metabolism of lipids which include almost all aspects of lipid metabolism like lipid digestion, transport, biosynthesis and catabolism [5]. Thyroid hormones stimulate the enzymes involved in the lipid metabolism such as lipoprotein lipase, hepatic lipase, Lecithin Cholesterol Acyltransferase (LCAT) and Cholesterol Ester Transfer Protein (CETP) [6]. Thyroid hormones along with other hormones (like insulin, cortisol, estrogen) play an important role in regulation of the activity of 3-Hydroxy-3-Methyl-Glutaryl Coenzyme A reductase (HMG CoA reductase) which is the rate limiting enzymes in cholesterol synthesis in the liver [7]. Thyroid hormones also regulate the enzyme cholesterol 7-hydroxylase, which is involved in the conversion of cholesterol into bile salts and this is the only pathway by which cholesterol can be excreted from the body [8]. Therefore, overt hypothyroidism is associated with dyslipidemia including increased Total Cholesterol (TC), Triglycerides (TG), High Density Lipoprotein (HDL) and Low Density Lipoprotein (LDL) resulting in increased risk of Coronary Artery Disease (CAD) in such patients. But the overall risk of cardiovascular disease cannot be explained purely on the basis of abnormal conventional lipid parameters because many times CAD occurs in patients who are normolipidemic [9]. This led us to change our focus towards the evaluation of non-conventional lipid parameters which can explain the risk of CAD in majority of the patients. Sd LDL and ox LDL are two such markers which are proven by many studies to better correlate with the risk and severity of CAD [10].
LDL is variable in structure, size and density. The LDL-C fraction can be divided into two fractions using graded gel electrophoresis. First fraction is small but dense and called as sd LDL and other fraction is larger and buoyant [11]. Studies have proved that this sd LDL fraction has higher tendency to get oxidized leading to their deposition in macrophages to form foam cells which are basis for the formation of atheromatous plaque. Therefore, patients who have higher proportion of sd LDL are at more risk of developing atherosclerosis even if they have normal LDL levels [12]. Hence, the amount of sd LDL fraction can explain the risk of CAD better than the total LDL levels. It is already proved that LDL is deposited inside the macrophages to form the foam cells after its oxidation by various processes. Therefore, the measurement of ox LDL is of greater importance for assessing the risk of the development of the atherosclerosis [13]. Taking into account the above facts we have done this study taking sd LDL and ox LDL in addition to conventional lipid parameters like TC, TG, HDL and LDL for assessing the risk of CAD in patients suffering from hypothyroidism. We also elucidated the association of serum TSH level with values of ox LDL, sd LDL and conventional lipid parameters.
Adding a new dimension of our existing knowledge, several studies have found an independent and continuous association between Lp (a) and cardiovascular diseases [14,15]. Lp (a) is a complex of low density lipoprotein in which apoB-100 is linked to apo (a) by a disulfide bridge. Lp (a) promotes foam cell formation and deposition of cholesterol, resulting in an increased atherosclerotic and thrombogenic potential. The mechanism may be related to the decreased clearance of Lp (a) mediated by the LDL-R degradation pathway [16]. The presence of apo (a) increases the density of Lp (a) compared with LDL-C and reduces its affinity for the LDL receptor. Lp (a) also promotes thrombosis by interfering with the fibrinolytic pathway [17].
Many studies have strongly elucidated that apo B estimation have come out to be better marker of risk of vascular disease and a better guide to adequacy of statin treatment than any cholesterol index [18]. Infact a larger study concluded that the non-fasting apo B/apo A1 ratio was superior to any of the cholesterol ratios for estimation of the risk of acute myocardial infarction in all ethnic groups, in both sexes and at all ages [19].
Therefore, we also included the evaluation of the levels of apo B and apo A1 in our study under the extended lipid profile and compared with that of the LDL and HDL in hypothyroid patients. Moreover owing to the association of Lp (a) with the cardiovascular risk, we also estimated its levels in hypothyroid patients and compared it with the conventional lipid profile.
Materials and Methods
This study was conducted in the Department of Biochemistry in a tertiary care hospital associated with a Medical College in Haryana for a period of 12 months (Jan 2014- Dec 2014). Prior to the commencement of the study, ethical clearance was taken from the ethical committee of the Institute. One hundred and thirty clinically proven patients of hypothyroidism aged 20-60 years were taken as cases. The diagnosis was based on detailed history, clinical examination, laboratory findings (increased TSH i.e. >6μIU/ml and decreased FT4 i.e. <4.1μg/ml respectively. One hundred and thirty age and gender matched healthy individuals from general population belonging to Haryana state were taken as controls. Patients were excluded from the study if they were using hypolipidemic drugs, oral contraceptives or hormone replacement therapy including thyroxine supplements. Patients with history of smoking, chronic alcoholism, chronic liver or kidney diseases and any concomitant acute or chronic infection were also excluded from the study.
After explaining the details and utility of the study, informed and written consent was taken from both cases and controls. Detailed history of both case and control group subjects was taken and clinical examination was performed. Details of laboratory investigations were noted for all the subjects.
The fasting samples (after overnight fasting of 12-14 hours), from cases and controls were taken in the morning taking all aseptic precautions from antecubital vein. The blood was centrifuged for 15 minutes at 3500rpm; and serum was separated and used for the estimation of routine lipid profile, ox LDL, Apo A1, Lp (a), sd LDL and Apo B. Lipid profile was done on fully automated Biochemistry analyser ‘EM 200’. TC was estimated by enzymatic end-point cholesterol esterase-peroxidase method (ERBA diagnostics Manheim GmbH, Germany). Triglycerides were estimated by enzymatic end-point glycerol phosphate oxidase-peroxidase method (ERBA diagnostics Manheim GmbH, Germany). HDL-C was estimated by direct Assay based on precipitation method where selective precipitation of LDL, VLDL and TG occurs and HDL is measured enzymatically by cholesterol oxidase and cholesterol esterase followed by Trinder reaction. LDL-C was estimated by direct assay based on modified Polyvinyl Sulfonic Acid (PVS) and Polyethylene-Glycol Methyl Ether (PEGME) coupled classic precipitation method. Ox LDL was estimated by ELISA kit (Elabscience Biotechnology Co., Ltd, Beijing) which is based on the direct sandwich technique in which two monoclonal antibodies are directed against separate antigenic determinants on the oxidized apolipoprotein B molecule. Lipoprotein (a) was estimated by one step sandwich ELISA method, using a specific monovalent anti-apo(a) antibodies (immunozym, ProgenBiotechnik GMBH, Germany). Apo A1 and Apo B were measured by ELISA method. sd LDL was calculated indirectly by using the formula given by Hattori et al., [20]:
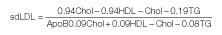
Statistical Analysis
The statistical analysis was performed using SPSS version 22.0, Minitab (Minitab Inc., PA) and Prism (Graphpad, San Diego, California, USA). Continuous variables were expressed as mean and standard deviation. Discrete variables were expressed as a percentage. Student’s t-test was applied to lognormal values of TSH, FT4, FT3, Lp (a), TG and Apo B as they followed lognormal distribution as proved by probability plots while t-test was applied directly to rest of the lipid values because they followed normal distribution. Differences were considered as significant if p-value was <0.05. Pearson’s correlation coefficient was calculated to find out the correlation between different parameters.
Results
The mean age of cases was 34.25 years and mean age of controls was of 37.23 years. As shown in the [Table/Fig-1], the number of females (98/130) was more than that of males (32/130) and it is evident that hypothyroidism was much more prevalent in females as compared to males (Female: Male ratio 3.06). Maximum number of patients in our study was in the age group of 31 to 40 years (63/130).
Age and sex distribution in cases and controls.
| Age(Years) | Controls(Total=130) | Cases(Total=130) |
|---|
| Males | Females | Males | Females |
|---|
| 21-30 | 05 | 10 | 02 | 10 |
| 31-40 | 25 | 40 | 10 | 53 |
| 41-50 | 10 | 23 | 12 | 20 |
| 51-60 | 06 | 11 | 08 | 15 |
| Total | 46 | 84 | 32 | 98 |
[Table/Fig-2] shows the normal values of TSH, FT4 and FT3 as well as the observed values in cases and controls in our study. Patient is said to be suffering from hypothyroidism when TSH value is >6.00μIU/ml and FT4 is <4.1μg/dl. We found that the TSH (p<0.0001) values were significantly high while that of FT4 (p<0.001) and FT3 (p<0.01) were significantly low in hypothyroid patients as compared to controls.
Thyroid profile in cases and controls.
| Parameter | Normal range | Controls (n=130)Mean ± SD | Cases (n=130)Mean ± SD | p-value |
|---|
| TSH(μIU/ml) | 0.39-6.00 | 3.12± 0.92 | 25.12± 8.92 | <0.0001 |
| FT4(μg/dl) | 4.1-12.00 | 6.79± 1.62 | 2.91± 1.78 | <0.001 |
| FT3(ng/ml) | 0.40-2.00 | 1.28± 0.31 | 0.61± 0.21 | <0.01 |
There was significant increase in the levels of ox LDL, sd LDL, Lp (a), Apo B, TC, TG and LDL-C in hypothyroid patients as compared to healthy controls [Table/Fig-3]. The levels of HDL-C and apoA1 were also found to be elevated in hypothyroid patients but the difference was insignificant in comparison with the healthy controls.
Comparison of all the lipid parameters studied in cases and control groups.
| Parameter | Controls (n=130)Mean ± SD | Cases (n=130)Mean ± SD | p-value |
|---|
| TC (mg/dl) | 124.43±12.45 | 180.53 ±20.13 | <0.01 |
| TG (mg/dl) | 140.21±18.24 | 176.34 ± 31.45 | <0.03 |
| LDL-C (mg/dl) | 70.15±10.88 | 120.25 ± 26.76 | <0.001 |
| HDL-C (mg/dl) | 47.67±4.70 | 49.10 ± 4.90 | >0.05 |
| ox LDL (U/L) | 17.22±4.79 | 48.12±4.27 | <0.0001 |
| Lp(a) (mg/dl) | 14.51±3.20 | 34.14 ± 7.16 | <0.001 |
| Apo A1 (mg/dl) | 121.44±9.47 | 124.41 ± 12.14 | >0.05 |
| ApoB (mg/dl) | 70.13±12.54 | 128.07 ± 15.11 | <0.001 |
| sd LDL** (no units) | 1.47±0.20 | 1.22±0.20 | <0.0001 |
**In the table, the value of sd LDL represents the size of this particle (as calculated by Hattori et al., [20]). The concentration of sd LDL is inversely relate to the size. As the size decreases, the concentration increases.
Among all lipid parameters under study; ox LDL and sd LDL have shown highest statistical significant difference (p<0.0001) while TG has shown lowest (p<0.03) among cases and control groups. This is an interesting finding as recent researchers are also turning their attention towards non-LDL cholesterol estimations as better predictors of cardiovascular risks as compared to LDL-C levels alone. It has already been established in some studies that patients with normal LDL-C levels as reported on the standard lipid panel; still have fairly large number of atherogenic particles like sd LDL and ox LDL.
To further consolidate our findings we compared the levels of advanced parameters like sd LDL, ox LDL, Apo B and Lp (a) in our study among the hypothyroid patients who had LDL-C levels in low as well as high cardiac risk categories. We divided all the hypothyroid patients of our study group in two categories according to the Adult Treatment Panel III (ATP III) guidelines given by National Cholesterol Education Program (NCEP) [21]. It was found that 80 out of 130 hypothyroid patients had LDL<130mg/dL (desirable range). Interestingly, we found an abnormal value of at least either of the advanced lipid parameters like ox LDL, sd LDL or apo B in such patients. Such values give an important insight; that even when the LDL-C levels are desirable; an abnormality in the advanced lipid parameter might exist. In order to draw more valuable information from our study, we further sub-divided all the hypothyroid patients of our study group into different ranges of the TSH and found that maximum number of patients who had abnormal levels of ox LDL or sd LDL were the ones who had very high TSH levels.
[Table/Fig-4] shows the values of the correlation coefficients of the conventional and non-conventional/advanced lipid parameters with the values of TSH. ox LDL has shown maximum correlation with the values of TSH (0.801) followed by sd LDL (0.792) and Apo B (0.783). It is evident from the [Table/Fig-4] that non-conventional parameters like ox LDL, sd LDL, Lp (a), Apo B and Apo A1 have shown better correlation with the hypothyroidism as compared to the conventional parameters such as TC, TG, LDL-C and HDL-C.
Correlation of different study parameters with TSH levels in hypothyroid patients.
| Parameter | r values | p-value |
|---|
| TC | 0.713 | <0.01 |
| TG | 0.684 | <0.03 |
| LDL-C | 0.741 | <0.001 |
| HDL-C | 0.731 | <0.001 |
| ox LDL | 0.801 | <0.0001 |
| Lp(a) | 0.709 | <0.001 |
| Apo A1 | 0.583 | <0.05 |
| ApoB | 0.783 | <0.001 |
| sd LDL | 0.792 | <0.0001 |
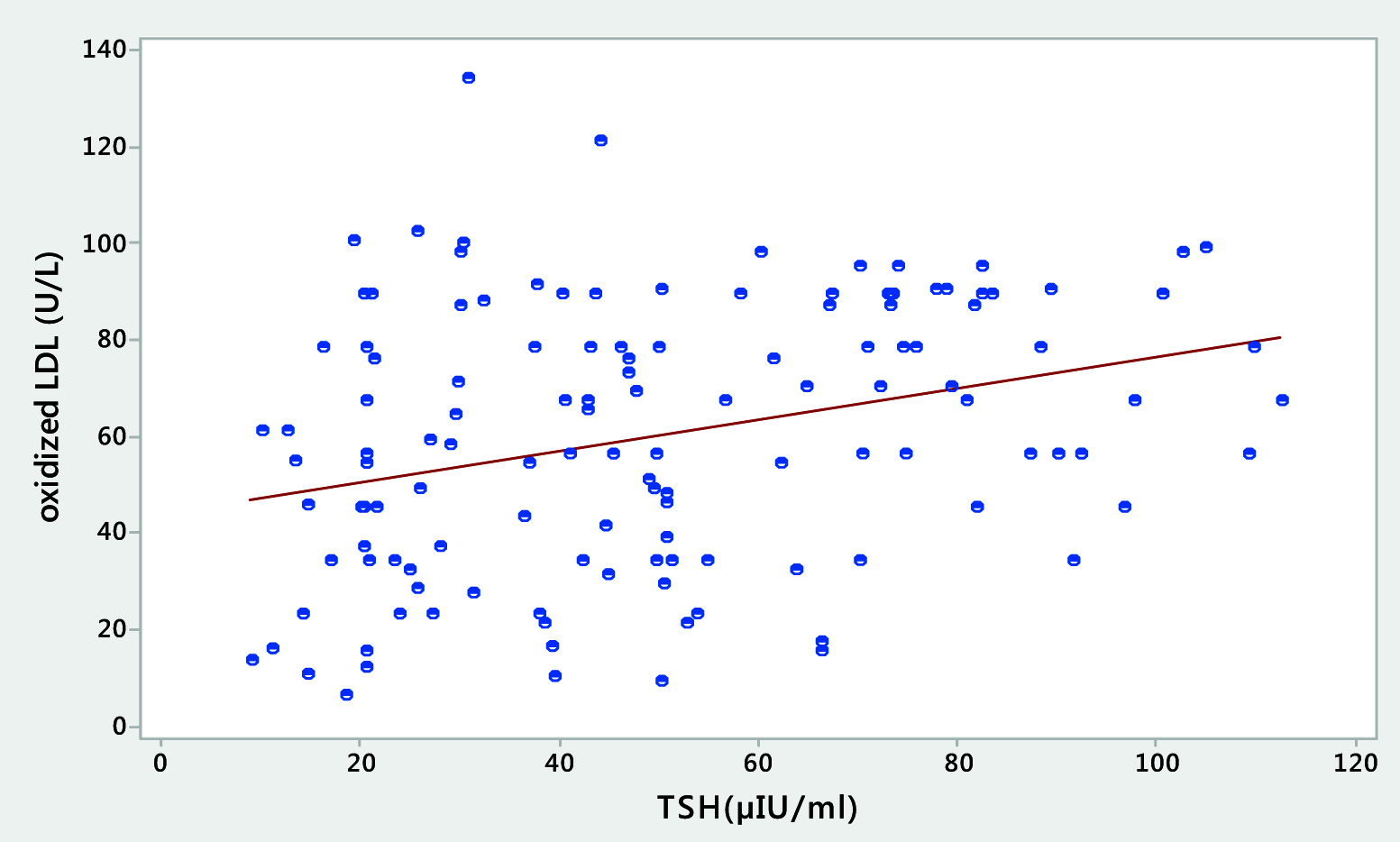
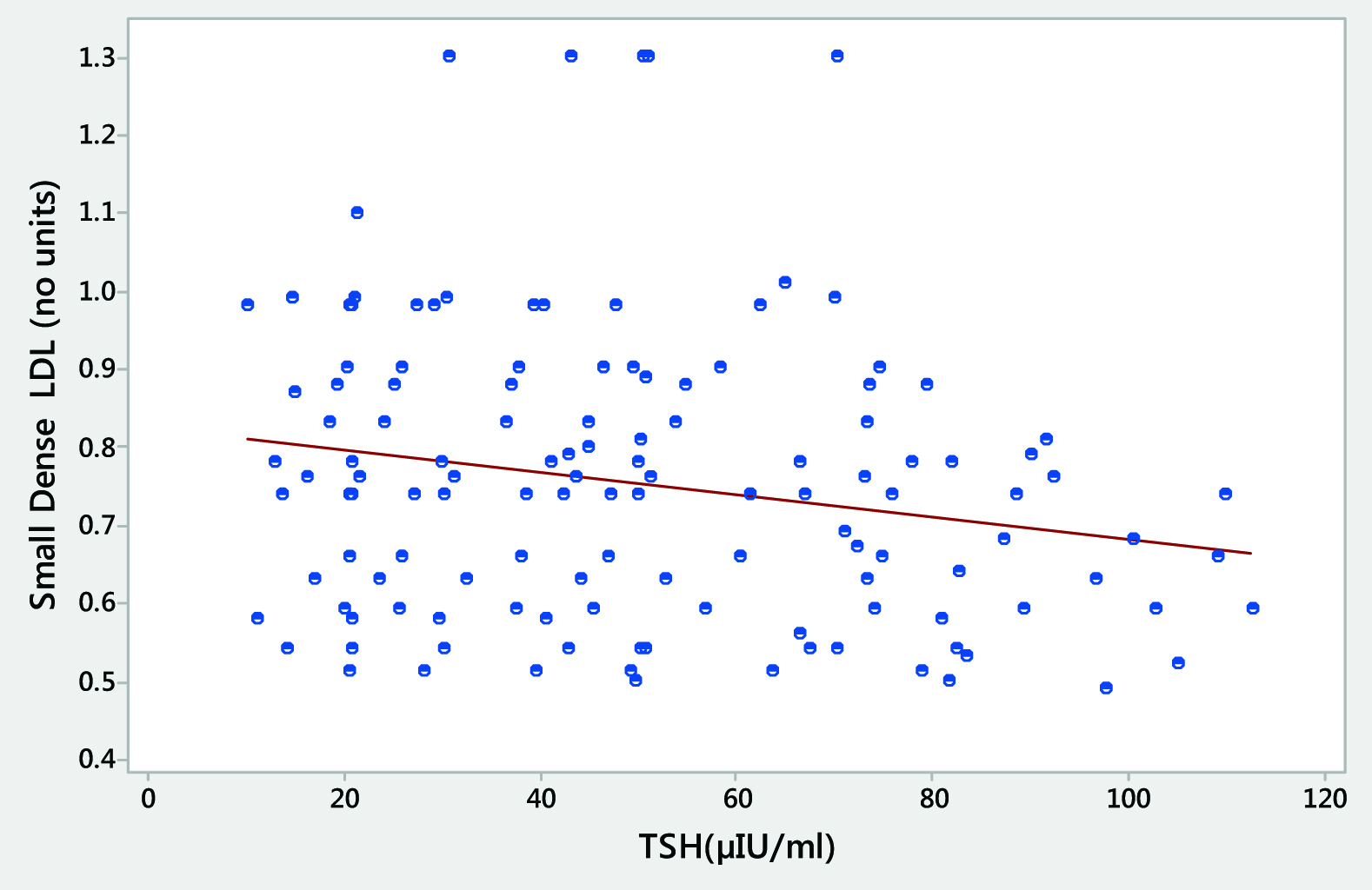
[Table/Fig-5,6] depict the scatter plot representations and clearly demonstrate the relationship of the most significant finding of our study that there is maximum correlation between the rising TSH values and the high ox LDL [Table/Fig-5] and also that increase in the TSH levels have a significant correlation with sd LDL particle and it becomes more and more heterogeneous in shape and size [Table/Fig-6].
Scatter plot representation showing a positive correlation between the levels of TSH and ox LDL in hypothyroid patients.
Scatter-plot representation showing significant correlation between the heterogeneity in shape and size of the sd LDL with the levels of TSH in hypothyroid patients.
Discussion
Hypothyroidism is the most common pathologic hormone deficiency among the endocrine disorders. Hypothyroidism may be due to primary disease of the thyroid gland itself or hypo-secretion of the pituitary TSH. Thyroid hormones have significant effects on synthesis, mobilisation and metabolism of lipids. Overt hypothyroidism is associated with significant hypercholesterolaemia [22].
Thyroid hormone regulates cholesterol synthesis through multiple mechanisms. Thyroid hormone affects some key components associated with cholesterol metabolism like HMG-CoA Reductase, Sterol Regulatory Element Binding Protein-2 (SREBP-2), LDL Receptor, ATP-Binding Cassette Transporters (ABCA1 and ABCG5/8) and 7-hydroxylase (rate limiting enzyme in bile acid synthesis which is responsible for maintaining whole body cholesterol homeostasis) at genetic level [23–28].
Moreover thyroid hormones affect the activity of the enzymes involved in the metabolism of lipoproteins and reverse cholesterol transport such as hepatic lipase, Cholesteryl-Esters Transfer Protein (CETP) and Lecithin-Cholesterol Acyltransferase (LCAT) [29]. In overt hypothyroidism, thyroid hormone effects on LDL receptor expression and cholesterol absorption will be suppressed and this in turn raises LDL levels. Additionally Lipoprotein Lipase (LPL) activity is decreased in hypothyroidism which results in higher TG and VLDL levels [29].
The complex interplay of these factors leads to raised total cholesterol levels in hypothyroid individuals. In our study we found a significant increase in the levels of total cholesterol, LDL, HDL cholesterol as well as TG concentration when the hypothyroid cases were compared with the controls. The raised serum LDL level is attributed to the downregulation of the hepatic LDL receptors under the effect of the hypothyroidism and thereby decreased fractional clearance of the LDL cholesterol despite of the simultaneous decreased activity of HMG CoA reductase enzyme [30]. In addition to this, we found slightly elevated levels of HDL cholesterol in hypothyroid individuals. This is actually due to increased concentration of HDL2 particles. Decreased activity of the CETP results in reduced transfer of the cholesteryl esters from HDL to VLDL, thus increasing the HDL cholesterol levels. Furthermore, decreased activity of the hepatic lipase leads to decreased catabolism of HDL2 particles [31]. This explains that there is a rise in HDL levels in hypothyroidism. Findings in our study have also been in accordance with this mechanism but the difference is insignificant when compared to healthy controls. Moreover significant rise in atherogenic particles like LDL, sd LDL and ox LDL outweighs the insignificant little increase in anti-atherogenic HDL, thereby putting the hypothyroid patients to cardiovascular risk.
Thyroid hormones regulate lipoprotein lipase, an essential enzyme responsible for removing TG from circulating chylomicrons and VLDL. Infact thyroid hormone regulation of the circulating TG level is more complex involving multiple pathways and positive and negative gene regulations [32]. Thus, there is significant hypertriglyceridemia seen in the hypothyroid group of the patients in our study also.
Raised LDL cholesterol concentration is an established risk factor for the development of cardiovascular diseases and therefore maintenance of its optimal levels has always been a primary target for the many lipid lowering drug regimens for decades. Recently evidences are increasing where a substantial percentage of patients with atherosclerotic vascular disease have LDL-C in the optimal range [33]. The reason for this mismatch is that the LDL particles are extremely heterogeneous with respect to the amount of cholesterol contained in its core. Thus, we measured sd LDL and ox LDL in the hypothyroid patients and compared with the controls and found that the levels of these two parameters were very highly significantly raised in the hypothyroid patients as compared to those in the control group. In addition to this, sd LDL and ox LDL showed maximum correlation with serum TSH levels in the hypothyroid group as compared to any other study parameters in the two groups of our study.
Interestingly, we found elevated levels of the sd LDL in patients of hypothyroidism who had optimal LDL cholesterol levels i.e. LDL<130mg/dL. However, the percentage of such individuals was less and a statistical significance could not be drawn; still it is an important finding and overcomes the limitation of considering only LDL as the predictor of cardiovascular risk. To consolidate the evaluation of the cardiovascular risk in hypothyroid patients, in our study we also measured and found a significantly high apoB levels in hypothyroid patients when compared to the healthy controls; which is considered as a key component of all the atherogenic lipoprotein particles including LDL, VLDL and IDL [34].
Thus, from our study it can be clearly elucidated that thyroid hormones affect the metabolism of the lipids in our body and hypothyroidism surely plays a vital role in the derangement of the lipid profile thereby manifesting in the form of hypercholesterolaemia, hypertriglyceridemia, raised LDL and HDL levels. However, the difference in the HDL cholesterol concentration and apoA1 was not significant between the cases and control groups in our study. But, certainly the cardiovascular risk of the patients are increased with this type of lipid profile and most significant correlation with the TSH was found in precisely involved fractions like sd LDL, apo B and ox LDL and we found that hypothyroid patients had very highly significantly elevated levels of these three parameters as compared to those in healthy controls. Sd LDL has been found to be raised in the conditions in which dyslipidemia is a feature like in diabetes mellitus and metabolic syndrome in many of the recent studies [35]. This sd LDL has maximum propensity to get oxidised and forming foam cells and leading on to atherosclerosis [36].
Lp(a) is an independent risk factor for the prediction of cardiovascular risk in the population. In our study the hypothyroid patients had significantly high levels of Lp(a) as compared to healthy controls. This Lp(a) promotes foam cell formation and deposition of cholesterol, resulting in increased atherosclerotic and thrombogenic potential. The mechanism may be related to the decreased clearance of Lp(a) mediated by the LDL receptor degradation pathway [37].
Conclusion
From our study, it might be imperative to propose that there is dyslipidemia in hypothyroidism and the extended lipid profile which includes parameters like sd LDL, ox LDL, apolipoproteins (apoB and apoA1) and Lp (a) along with the conventional lipid profile has a superiority in predicting the cardiovascular risk in such patients. This will pave the gap towards the underestimation of such risk in thyroid disorders especially who might present with optimal LDL cholesterol levels. Therefore, the chances of missing the diagnosis of the individuals with a risk of CAD would become least and prompt management would be started.
**In the table, the value of sd LDL represents the size of this particle (as calculated by Hattori et al., [20]). The concentration of sd LDL is inversely relate to the size. As the size decreases, the concentration increases.
[1]. Boucai L, Hollowell JG, Surks MI, An approach for development of age, gender and ethnicity specific thyrotropin reference limitsThyroid 2011 21(1):5-11. [Google Scholar]
[2]. Gaitonde DY, Rowley KD, Sweeny LB, Hypothyroidism: an updateS Afr Form Pract 2012 3:84-90. [Google Scholar]
[3]. Devdhan M, Ousman YH, Burman KD, HypothyroidismEndocrinol Metab Clin North Am 2007 36(3):595-615. [Google Scholar]
[4]. Dutta P, Bhansali A, Masood SR, Bhadada S, Sharma N, Rajput R, Predictors of outcome in myxedema coma: A study from a tertiary care centreCrit Care 2008 12(1):R1 [Google Scholar]
[5]. Ladenson PW, Singer PA, Ain KB, American thyroid association guidelines for detection of thyroid dysfunctionArch Intern Med 2000 160(11):1573-75. [Google Scholar]
[6]. Asvold BO, Vatten LJ, Nilsen TI, Bjoro T, The association between TSH within the reference range and serum lipid concentrations in a population based study. The HUNT studyEur J Endocrinol 2007 156:181-86. [Google Scholar]
[7]. Santamarina-Fojo S, Gonzalez-Novarro H, Freeman L, Wagner E, Nong Z, Hepatic lipase, lipoprotein metabolism and atherogenesisArterioscler Thromb Vasc Biol 2004 24:1750-54. [Google Scholar]
[8]. Rensen PC, VanDijk KW, Havekes LM, Apolipoprotein A: low concentration, high impactArterioscler Thromb Vasc boil 2005 25:2445-47. [Google Scholar]
[9]. Pearce EN, Wilson PW, Yang Q, Vasan RS, Braverman LE, Thyroid function and lipid sub-particle sizes in patients with short-term hypothyroidism and a population based cohortJ Clin Endocrinol Metab 2008 93:888-94. [Google Scholar]
[10]. Sharma SB, Garg S, Small dense LDL: Risk factor for Coronary Artery Disease (CAD) and its therapeutic modulationIndian J Biochem Biophys 2012 49:77-85. [Google Scholar]
[11]. Kim CS, Kang JG, Lee SJ, Relationship of low density lipoprotein (LDL) particle size to thyroid function status in KoreansClin Endocrinol 2009 71:130-36. [Google Scholar]
[12]. Rizos CV, Elisaf MS, Liberopoulos EN, Effects of thyroid dysfunction on lipid profileThe Open Cardiovascular Med J 2011 5:76-84. [Google Scholar]
[13]. Chinche F, Jublanc C, Coudert M, Carreau V, Kahn JF, Bruckert E, Hypothyroidism is not associated with increased carotid atherosclerosis when cardiovascular risk factors are accounted for in hyperlipidemic patientsAtherosclerosis 2009 203:269-76. [Google Scholar]
[14]. Jacobson TA, Lipoprotein(a), cardiovascular diseases and contemporary managementMayo Clin Proc 2013 88:1294-311. [Google Scholar]
[15]. Nordestgaard BG, Chapman MJ, Ray K, Lipoprotein(a) as a cardiovascular risk factor: Current statusEur Heart J 2010 31:2844-53. [Google Scholar]
[16]. Galman C, Bonde Y, Matasconi M, Angelin B, Rudling M, Dramatically increased intestinal absorption of cholesterol following hypophysectomy is normalized by thyroid hormonesGastroenterology 2008 134:1127-36. [Google Scholar]
[17]. Lippi G, Guidi G, Lipoprotein(a): An emerging cardiovascular risk factorCrit Rev Clin Lab Sci 2003 40:1-42. [Google Scholar]
[18]. Sniderman AD, Furberg CD, Keech A, Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatmentLancet 2003 361(9359):777-80. [Google Scholar]
[19]. McQueen MJ, Hawken S, Wang X, Lipids, lipoproteins and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control studyLancet 2008 372(9634):224-33. [Google Scholar]
[20]. Hattori Y, Development of approximate formula for LDL-chol, LDL-apo B and LDL/LDL-Apo B as indices of hyperApoBetalipoproteinemia and small dense LDLAtherosclerosis 1998 138(2):289-99. [Google Scholar]
[21]. Expert Panel on Detection, Evaluation and Treatment of high blood cholesterol in adultsExecutive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III)JAMA 2001 285:2486-97. [Google Scholar]
[22]. Cappola AR, Ladenson PW, Hypothyroidism and atherosclerosisJournal of Clinical Endocrinology. Metabolism 2003 88:2438-44. [Google Scholar]
[23]. Ness GC, Chambers CM, Feedback and hormonal regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase: the concept of cholesterol buffering capacityProc Soc Exp Biol Med 2000 224:8-19. [Google Scholar]
[24]. Goldstein JL, DeBose-Boyd RA, Brown MS, Protein sensors for membrane sterolsCell 2006 124:35-46. [Google Scholar]
[25]. Shin DJ, Osborne TF, Thyroid hormone regulation and cholesterol metabolism are connected through Sterol Regulatory Element- Binding Protein-2 (SREBP-2)J Biol Chem 2003 278:34114-18. [Google Scholar]
[26]. Lopez D, Abisambra SJF, Bedi M, Ness GC, Activation of the hepatic LDL receptor promoter by thyroid hormoneBiochim Biophys Acta 2007 1771:1216-25. [Google Scholar]
[27]. Huuskonen J, Vishnu M, Pullinger CR, Fielding PE, Fielding CJ, Regulation of ATP-binding Cassette transporter A1 transcription by thyroid hormone receptorBiochemistry 2004 43:1626-32. [Google Scholar]
[28]. Hashimoto K, Cohen RN, Yamada M, Markan KR, Monden T, Satoh T, Cross-talk between thyroid hormone receptor and liver X receptor regulatory pathways is revealed in a thyroid hormone resistance mouse modelJ Biol Chem 2006 281:295-302. [Google Scholar]
[29]. Jin T, Teng X, Update on lipid metabolism and thyroid disordersJ Endocrinol Diabetes Obes 2014 2(3):1043 [Google Scholar]
[30]. Leonidas H, Duntas Thyroid disease and lipidsThyroid: Official Journal of the American Thyroid Association 2002 12(4):287-93. [Google Scholar]
[31]. Liberopoulos EN, Elisaf MS, Dyslipidemia in patients with thyroid disordersHormones 2002 1(4):218-23. [Google Scholar]
[32]. Calandra S, Priore Oliva C, Tarugi P, Bertolini S, APOA5 and triglyceride metabolism, lesson from human APOA5 deficiencyCurr Opin Lipidol 2006 17:122-27. [Google Scholar]
[33]. Sachdeva A, Cannon CP, Deedwania PC, lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get with the GuidelinesAm Heart J 2009 157(1):111-17. [Google Scholar]
[34]. Brunzell JD, Davidson M, Furberg CD, Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology FoundationJ Am Coll Cardiol 2008 51(15):1512-24. [Google Scholar]
[35]. Halpern Metabolic syndrome, dyslipidemia, hypertension and type 2 diabetes in youth: From diagnosis to treatmentDiabetology and Metabolic Syndrome 2010 2:55-75. [Google Scholar]
[36]. Mitra S, Oxidised low-density lipoprotein and atherosclerosis implications in antioxidant therapyAm J Med Sci 2011 342(2):135-42. [Google Scholar]
[37]. Galman C, Bonde Y, Matasconi M, Angelin B, Rudling M, Dramatically increased intestinal absorption of cholesterol following hypophysectomy is normalized by thyroid hormoneGastroenterology 2008 134:1127-36. [Google Scholar]