Illustrated Imaging Essay on Congenital Heart Diseases: Multimodality Approach Part II: Acyanotic Congenital Heart Disease and Extracardiac Abnormalities
Venkatraman Bhat1, Vinay Belaval2, Karthik Gadabanahalli3, Vimal Raj4, Sejal Shah5
1 Director of Imaging Services, Head of Radiology, Department of Radiology and Imaging services, Narayana Health, Narayana Hrudayalaya, Multispeciality Hospital-Shaw Mazumdar Medical Centre, Bengaluru, India.
2 Junior Consultant, Department of Radiology and Imaging services, Narayana Health, Narayana Hrudayalaya, Multispeciality Hospital-Shaw Mazumdar Medical Centre, Bengaluru, India.
3 Consultant Radiology, Department of Radiology and Imaging services, Narayana Health, Narayana Hrudayalaya, Multispeciality Hospital-Shaw Mazumdar Medical Centre, Bengaluru, India.
4 Consultant Radiology, Department of Radiology and Imaging services, Narayana Health, Narayana Hrudayalaya, Multispeciality Hospital-Shaw Mazumdar Medical Centre, Bengaluru, India.
5 Senior Consultant Paediatric Cardiology, Department of Pediatric cardiology, Narayana Health, Narayana Hrudayalaya, Bengaluru, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Venkatraman Bhat, 309, Greenwoods Apartment Royal Gardenia, Bommasandra, Bangalore-560099, India.
E-mail: bvenkatraman@gmail.com
Acyanotic heart disease constitutes a significant majority of patient who may present with non-cardiac symptoms. Either they are detected incidentally or present with respiratory complaints. Equipped with knowledge of anatomy by echocardiography and radiographic methods described in previous part of this presentation, diagnosis may be confidently attempted. On plain radiography acyanotic congenital heart diseases have variable appearance depending upon severity of disease. Cardiac size, chamber enlargement and pulmonary vascular pattern are key elements. Typically left to right shunts with large volume flow are associated with pulmonary plethora. Plain radiography has an important role in detecting manifestation of pulmonary arterial hypertension. Severe stenosis of pulmonary valve is associated with pulmonary oligemia. Small intra-cardiac shunts and anomalies of coronary arteries generally present with normal cardiac size and pulmonary arterial pattern. Disease spectrum presented in this illustration demands thorough scrutiny of pulmonary, osseous and abdominal abnormalities. This section illustrates some commonly encountered spectrum of acyanotic cardiac disease.
Absent right pulmonary artery, Anomalous pulmonary venous drainage, Coronary A-V fistula, Coarctation of aorta, Imaging
Introduction
This section on spectrum of acyanotic cardiac diseases is presented under following headings:
1. Case Illustrations: Consisting of-Atrial Septal Defect (ASD) and Ventricular Septal Defects (VSD), Patent Ductus Arteriosus (PDA), Partial Anomalous Pulmonary Venous Connections (PAPVC)
2. Miscellaneous Category Consisting of Coronary anomalies, Coarctation of aorta, valvular stenosis and Heterotaxy.
Septal Defects and Patent Ductus Arteriosus
Septal defects at ventricular, atrial level and ductus arteriosus can occur in isolation or in association with other complex heart disease. Clinical examination, echocardiography and plain radiography generally provide necessary information for management. Plain radiography provides information about lung parenchyma in addition to pulmonary plethora in association of large volume overload. L-R Shunting of more than 2-2.5 times is necessary to be visible as enlarged pulmonary vasculature on radiography. Early signs of pulmonary arterial hypertension should be looked for in suspected left-to-right shunts [1]. Echocardiography plays a key role in evaluation, complemented by MRI or CT in specific contexts [2,3]. Ventricular septal defects, invariably the most common congenital heart defect, manifest at earlier age than ASD, which can vary greatly in size and location [4]. Patent ductus is the natural pathway of left to right shunt, seen in association of many CHD and aortic coarctation. MRI or CT may add further information in complicated atrial septal defects or detect an occasional ductus missed on echocardiography. Abnormal pulmonary veins may be seen in association with atrial and ventricular septal defects detected either by plain radiography or CT evaluation. Quantification of functional parameters like cardiac output, ejection fraction, stroke volume, measurement of pressure gradient, regurgitant fraction and Qp to Qs can be done with MRI. Details of individual shunts are provided in [Table/Fig-1,2 and 3] [5–7].
Atrial septal defect: Important Facts [5].
| Subtypes | Ostium primum | Ostium secundum | Sinus venosus type | Coronary sinus type (unroofed CS) |
|---|
| SVC type | IVC type |
|---|
| Incidence | 1 child per 1500 live births. PFO are quite common (appearing in 10–20% of adults). Secundum ASD-most common. M:F 1:2 |
| Association | MSX1 is strongly associated with the risk of an ASDNKX2.5 gene implicated in non-syndromic ASDs.Heterotaxy syndrome, Eisenmenger’s Syndrome |
| Syndromes | Lutembacher’s syndrome (MS + ASD). Holt-Oram syndrome (Heart-Hand syndrome= upper limb malformations +ASD).
|
| Imaging Features (Plain) | Cardiomegaly | RA/ RV enlargement | Dilated PA | Pulmonary Plethora | |
| Imaging Features (Specific) | Echo-Direct demonstration of defect and jet MRI demonstration of extracardiac anomalies; shunt fraction; Qp:Qs estimation.
|
| Management | Transcatheter occlusion by diaphragm (only suitable for secundum ASD). Surgical ASD closure.
|
Ventricular Septal Defect: Important Facts [5].
| Subtypes | Membranous (80%) | Outlet (infundibular/ supracristal/subarterial/ subpulmonary or conal) (5-7%) | Inlet (AV canal type) (8%) | Trabecular (muscular) |
|---|
| Central/ apical/ marginal/ “swiss cheese” type (5-20%) |
|---|
| Incidence | 1.3-2.5 per 1000 live births. (4)Membranous VSD-most common. With paternal VSDs, the recurrence risk in an offspring is 2%. Maternal VSDs have a recurrence risk of 6% to 10 % [6]. No sexual preference. |
| Association | No specific gene implicated.Heterotaxy syndrome, Eisenmenger’s Syndrome |
| Syndromes | Chromosomal anomalies {trisomy 13,18,21; Cri du Chat syndrome (chromosome 5 short arm deletion)}; Noonan syndrome; VATER anomalies. |
| Imaging Features (Plain) | Cardiomegaly | LA/ LV enlargement | Dilated PA | Pulmonary Plethora |
| Imaging Features (Specific) | Echo-Direct demonstration of defect, prolapse of aortic valve cusp in outlet VSD and deformity of aortic cusp in membranous type. MRI demonstration of extracardiac anomalies; shunt fraction; Qp:Qs estimation.
|
| Management | Spontaneous closure of small muscular VSDs Transcatherter device closure Surgical closure.
|
Patent Ductus Arteriosus: Important Facts [2].
| Krichenko Angiographic Classification [6] | Type A(conical) | Type B (window) | Type C(tubular) | Type D(complex) | Type E(elongated) |
|---|
| Incidence | 1 in 2500 to 5000 live births. (7) M:F 1:3. |
| Association | Duct-dependent conditions (hypoplastic left heart syndrome, interrupted aortic arch); Pulmonary atresia. |
| Syndromes | Chromosomal aberrations (trisomy 21 and 4p- syndrome), single-gene mutations (Carpenter’s syndrome and Holt-OramSyndrome), X-linked mutations (incontinentia pigmenti). Char syndrome (an inherited disorder with PDA, facial dimorphism and hand anomalies) |
| Imaging Features Plain | Cardiomegaly | Pulmonary Plethora | Prominent ascending aorta and arch | Focal aortic dilatation (ductus bump) | LA enlargement |
| Imaging Features Specific | Echo Colour Doppler more usefulMRI for defining anatomy in unusual PDA geometry and in associated anomalies of aortic arch (right aortic arch, cervical arch)CT can assess degree of calcification in adult PDA[2,5] |
| Management | Transcatherter closure (Small PDA < 4mm Gianturco stainless coils; larger PDA Amplatzer device) [3,5]Surgical ligation (though thoracotomy or video-assisted thoracoscopic ligation) |
Details of Atrial Septal Defect (ASD) and imaging appearances is presented in [Table/Fig-1] and illustrated in [Table/Fig-4,5]. [Table/Fig-2,6] provides information and images of ventricular septal defect (VSD). [Table/Fig-3,7] provides information and images of Patent Ductus Arteriosus (PDA). Example of patent ductus arteriosus with severe pulmonary arterial hypertension and bronchial compression is shown in [Table/Fig-8].
A 13-year-old male patient presenting with exertional dyspnoea, diagnosed with atrial septal defect on echocardiography and MDCT: (a) Diagrammatic representation of secondum of atrial septal defect; (b) Plain radiograph shows mild cardiac enlargement, increased pulmonary arterial markings (arrows) and mildly dilated main pulmonary arterial segment(open arrow).
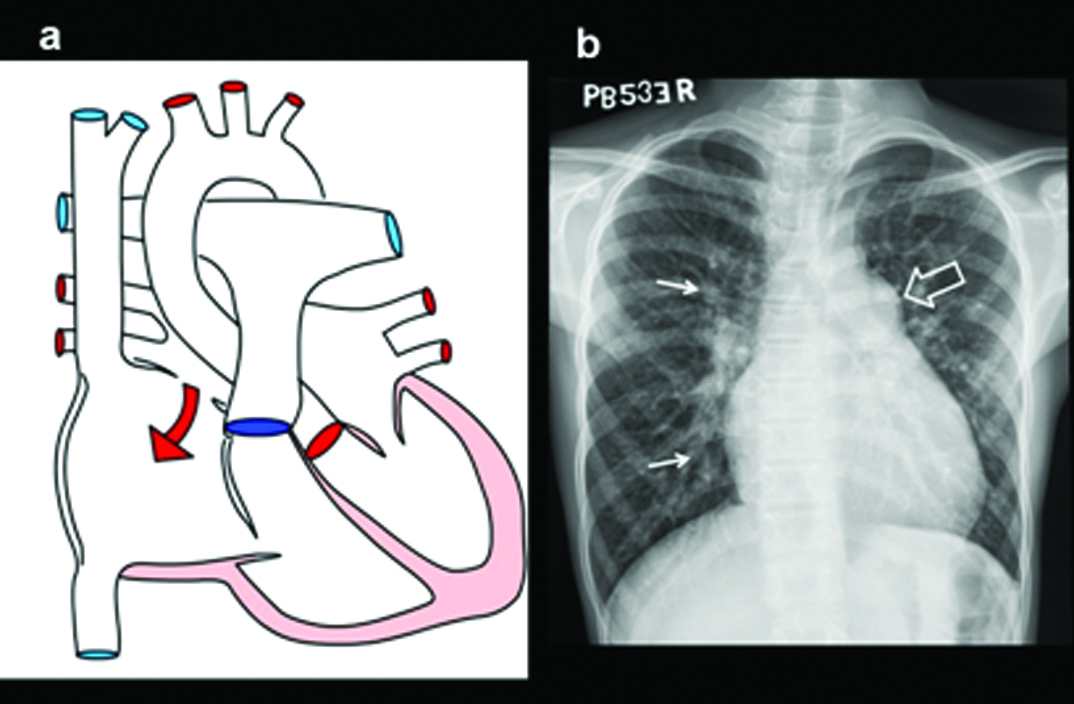
(c) Grey scale image of the right atrium illustrates sinus venosus ASD (arrow) (d) Colour Doppler image of the right atrium illustrates sinus venosus type of ASD (arrow) with the anomalous upper lobe vein (e) Reconstructed CT 4-CH view shows secundum ASD (arrows). There is enlargement of right atrium and ventricle (star); (f) 4-CH MR image in another patient shows a large septal defect (triangles).
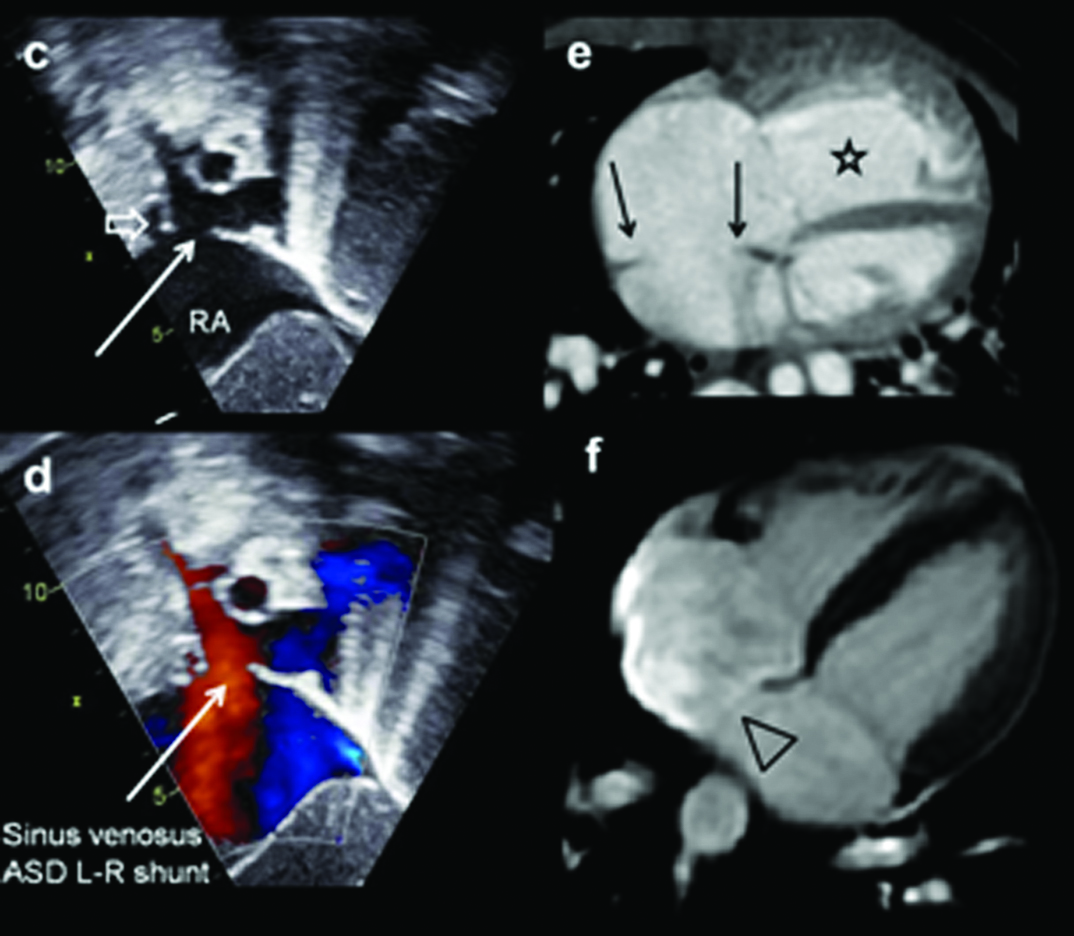
Six-month-old male patient diagnosed with a left-to-right shunt, VSD, confirmed on echocardiography: (a) Diagrammatic representation of ventricular septal defect; (b) Chest radiograph shows pulmonary plethora (open arrow) and moderately dilated main pulmonary arterial segment (arrow). There is right upper lobe collapse; (c) Grey scale and the colour doppler images of the right ventricular outflow shows the perimembranous septal defect (arrow) and the jet illustrated by the Doppler examination; (d) Axial CT view shows grossly dilated pulmonary artery (arrow); (e) Coronal CT reconstruction demonstrates enlarged left atrium (star). Increased arterial shadows; (f) Demonstrates peri-membraneous ventricular septal defect in 4CH MRI examination (open arrow).
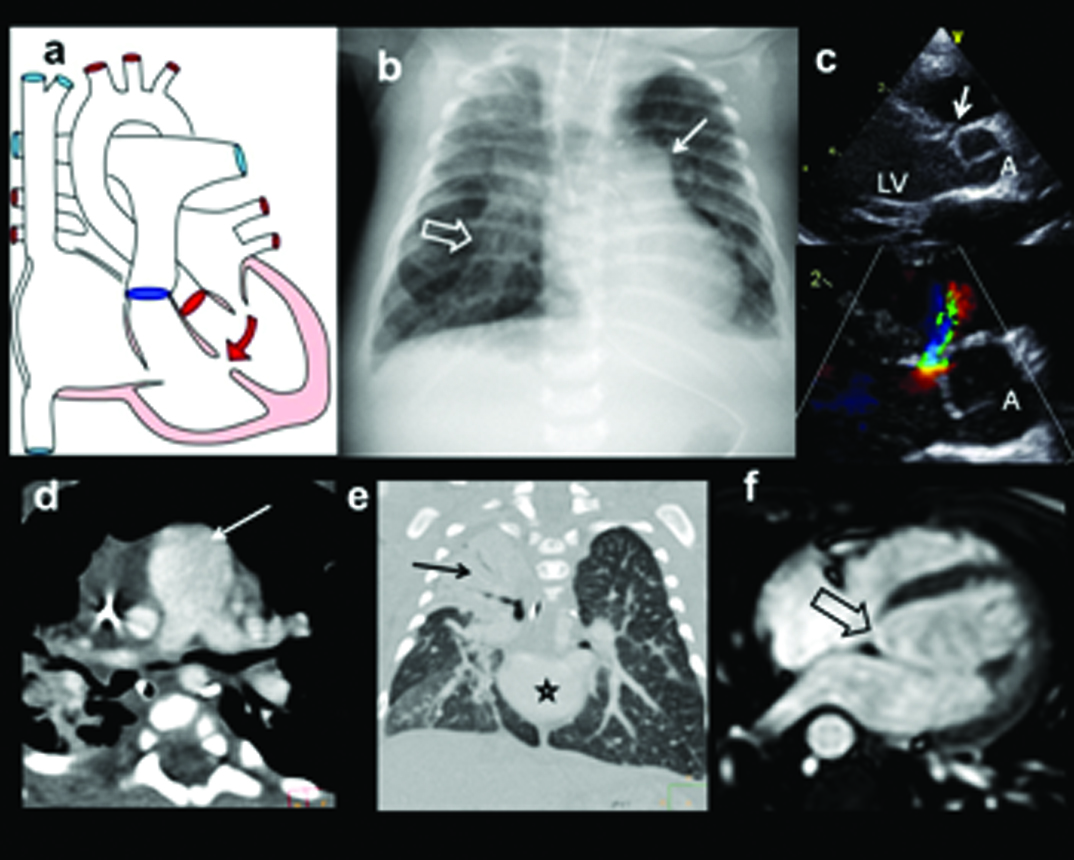
Eight-month-old, female diagnosed with patent ductus arteriosus on echocardiography, confirmed on MDCT: (a) Diagrammatic representation of patent ductus; (b) Plain radiograph shows moderate cardiac enlargement, increased pulmonary arterial markings, prominent aortic knuckle and left atrial enlargement (arrow); (c) Short axis Doppler view at the pulmonary arterial level shows the turbulent flow in the region of ductus (arrow). Pulmonary artery is noted medially (triangle). The continuous flow pattern is illustrated in the jet (C1). MDCT coronal (d) and axial; (e) views shows redundant patent ductus arteriosus (open arrows); (d) Size and length of ductus can vary considerably; (f) 3-D MDCT reconstruction demonstrates large ductus (angled arrow) connecting LPA and aorta.
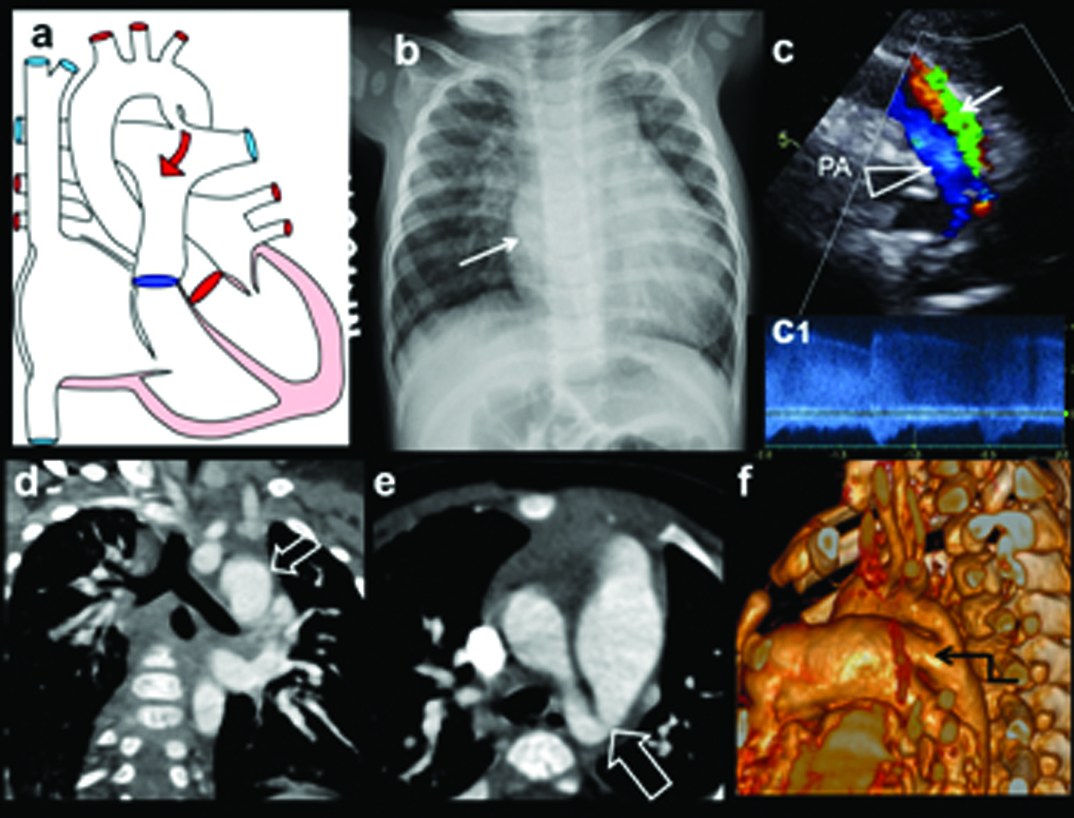
L-R shunt with PAH, bronchial compression:
Six-month-old female with feeding difficulty, irritability diagnosed with VSD, PDA with PAH. Echo demonstrated dilated MPA and VSD, PDA, PAH: (a) Plain radiograph shows moderate cardiomegaly with prominent MPA and proximal RPA (arrows); (b) axial CT image shows grossly dilated MPA and branches (arrows); (c) Coronal MIP CT image demonstrates, grossly dilated RPA; (d) Sagittal MIP images demonstrates severe dilatation of MPA and LPA (arrows); (e) 3D reconstruction of airway shows left bronchial compression(open arrow) by enlarged LPA.
Partial Anomalous Pulmonary Venous Connections (PAPVC)
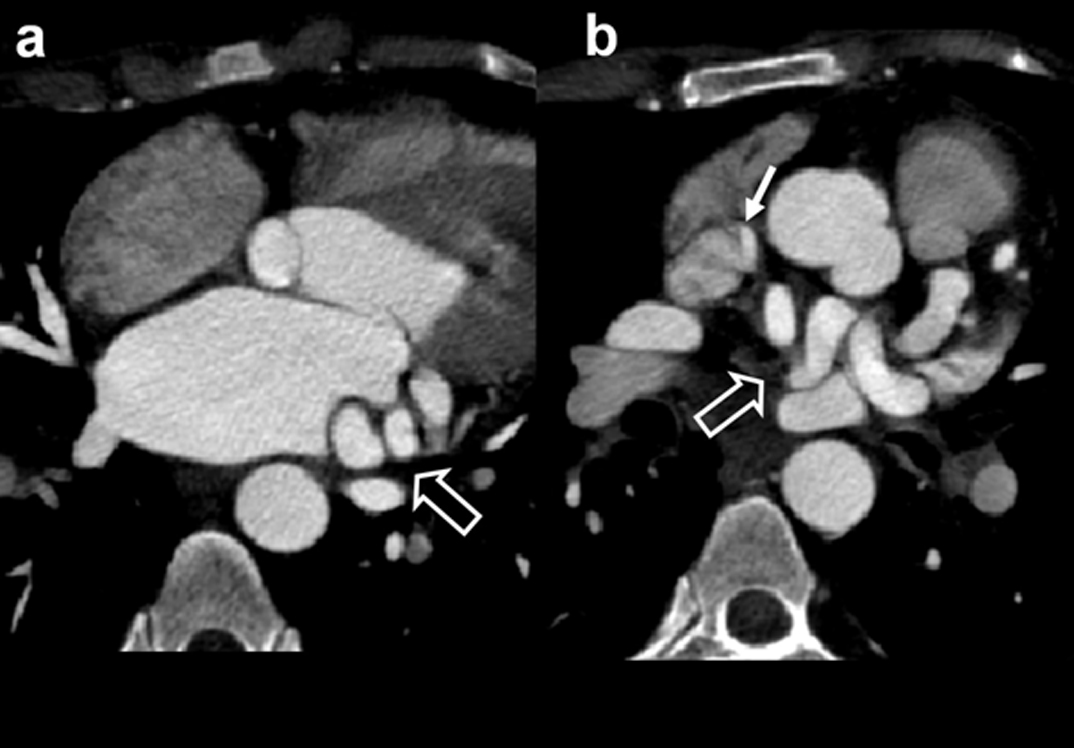
These groups of patients also come under the minor cardiac disease category due to small shunt volume. Partial pulmonary anomalous veins are often found incidentally or in association with other cardiac anomalies like ASD [5]. When shunt is small there are no significant hemodynamic changes. In larger connections significant left-right-shunting may occur. Pulmonary hypoplasia and pulmonary sequestration are associated with anomalous venous drainage. There is a greater role for cross-sectional imaging (CT/MRI) in detection of presence, location of anomalous veins and information about lung hypoplasia or sequestration. Diagnosis can often be obtained with single conclusive examination. Details are provided in [Table/Fig-9]. [Table/Fig-10,11] illustrates an example of Partial Anomalous Pulmonary Venous Connection (PAPVC) presenting with features of Scimitar syndrome. An additional example of partial anomalous pulmonary venous connection of superior and inferior pulmonary veins is shown in [Table/Fig-12,13 and 14]. PAPVC of superior pulmonary vein is presented in [Table/Fig-15].
PAPVC-Superior and inferior pulmonary veins: Important Facts.
| PAPVC | One or more (but not all) pulmonary veins drain into RA or its venous tributaries like SVC, IVC, left innominate vein and coronary sinus [5] |
| Subtypes | Left pulmonary veins drain either into left innominate vein or coronary sinus. Associated with ASD.Right pulmonary veins may drain into SVC (associated with sinus venosus ASD) or into IVC (Scimitar syndrome or venolobar sequestration) |
| Incidence | Seen in <1% of all CHDs. |
| Association | ASDBroncho-pulmonary sequestration (scimitar syndrome) |
| Syndromes | Scimitar syndrome = right PAPVC + hypoplastic right lung + hypoplastic RPA + right lower lobe sequestration |
| Imaging Features (Plain) | Cardiomegaly- RA/RV dilatation | Dilated SVC | “Scimitar sign” produced by an anomalous pulmonary vein that drains any or all of the lobes of the right lung. Seen as crescent shaped vertical shadow, curves outward along the right cardiac border, usually from the middle of the lung to the cardiophrenic angleResembles Turkish/Persian sword (=scimitar) |
| Imaging Features (Specific) | Echo direct demonstration of PAPVC; inability to visualize all pulmonary veins with RA/RV dilatation – strongly suggests PAPVCMRI in unclear pulmonary venous anatomyCT preferred in scimitar syndrome. |
| Management | Surgical correction (ligation & re-anastamosis) |
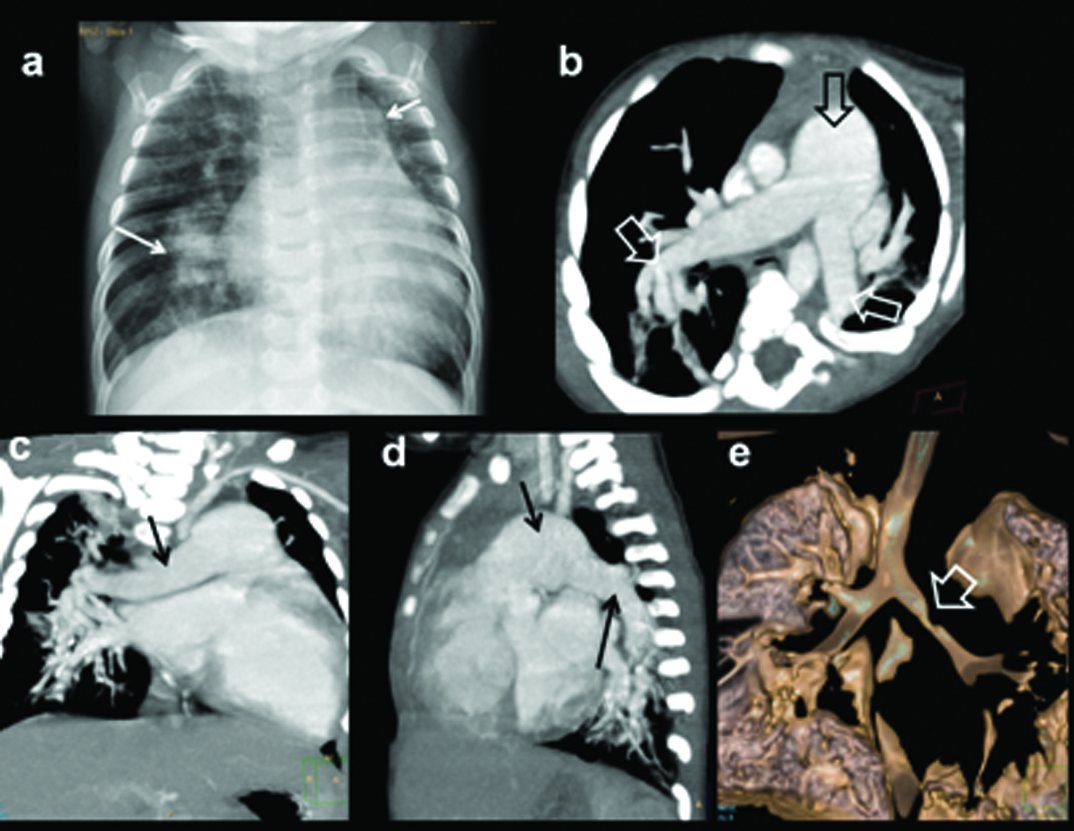
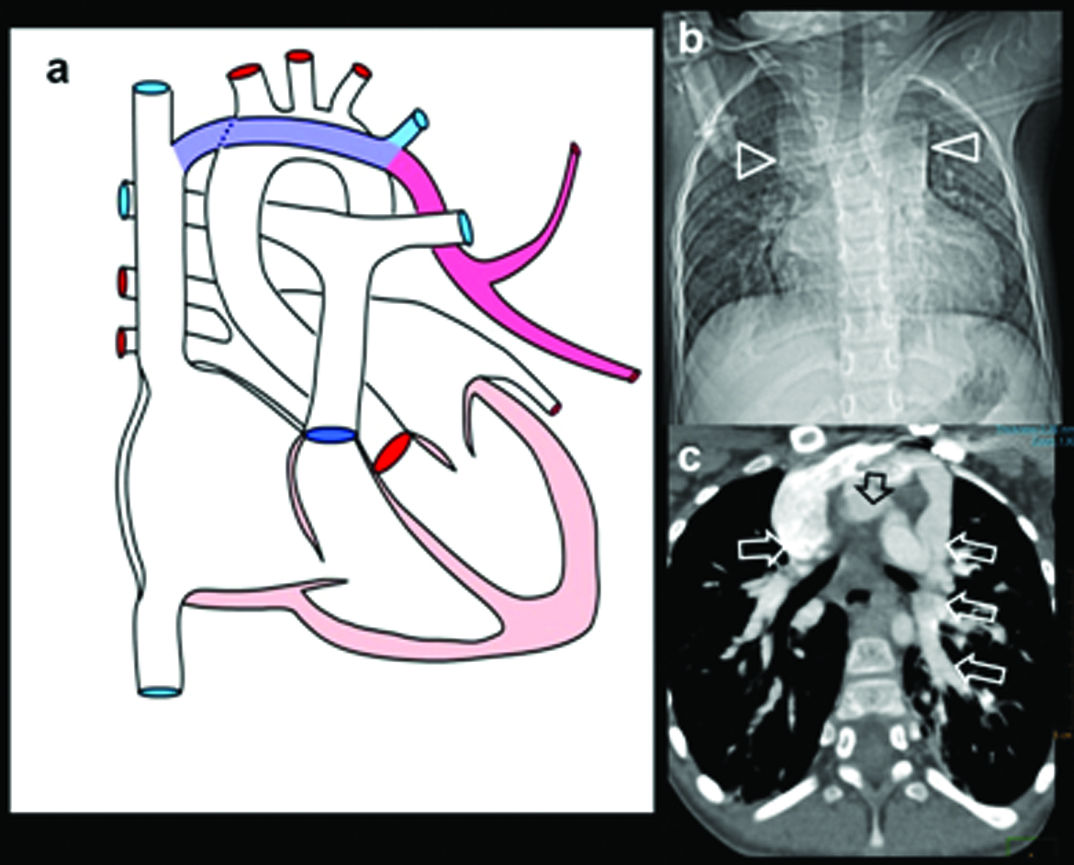
Six-month-old female presenting with recurrent chest infection, abnormal chest radiograph, diagnosed with PAPCV: (a) Diagrammatic representation of vascular anatomy in infra-cardiac PAPVC. Anomalous right inferior pulmonary vein is shown draining IVC (red); (b) Plain radiograph shows dextro-positon of cardiac shadow with increased pulmonary arterial markings in left lung. Right lung is hypoplastic; (c) Coronal oblique CT and sagittal; (d) reconstruction shows anomalous right inferior pulmonary vein joining distal IVC (arrow). Sequestrated lung is seen at right lung base (solid arrow).
(e) 3D CT image shows anomalous vein joining IVC (triangle); Additional 3D CT image in oblique view shows union of anomalous vein. Arteries from SMA supplying sequestrated lung segment (open arrows).
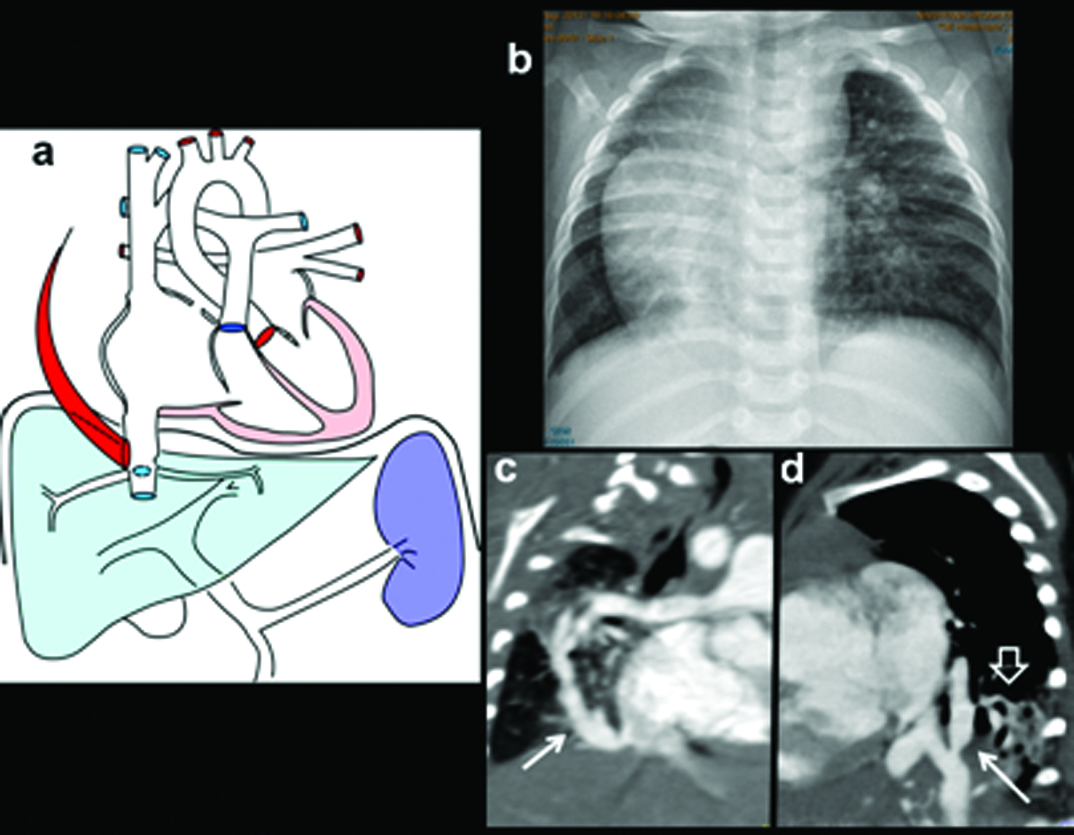
One-year-old female admitted to hospital with severe chest infection, breathlessness diagnosed with PAPVC: (a) Diagrammatic representation of vascular anatomy in infra-cardiac PAPVC. Anomalous right inferior pulmonary vein is shown draining IVC (colored red); (b) Coronal image shows anomalous pulmonary vein (arrow) with increased pulmonary arterial markings.
(c) Coronal CT reconstruction shows anomalous right inferior pulmonary vein joining distal IVC (triangle); (e) Axial CT images demonstrate a large secundum atrial septal defect (black arrow). There is biventricular and right atrial enlargement arrow; There are consolidative changes in sequestrated lower lobe (triangle) (d, f) Sagittal CT MIP image and 3D images demonstrates anomalous pulmonary vein (arrow).
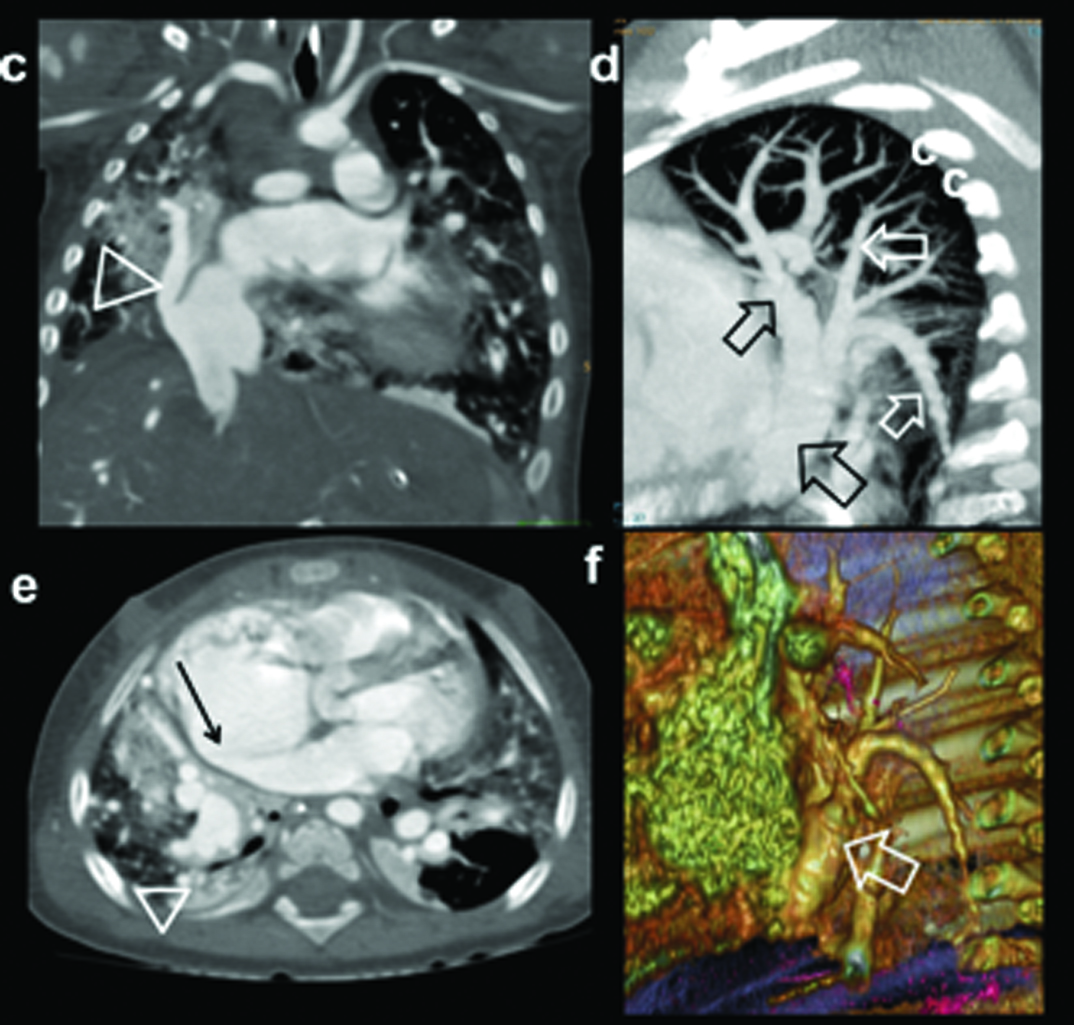
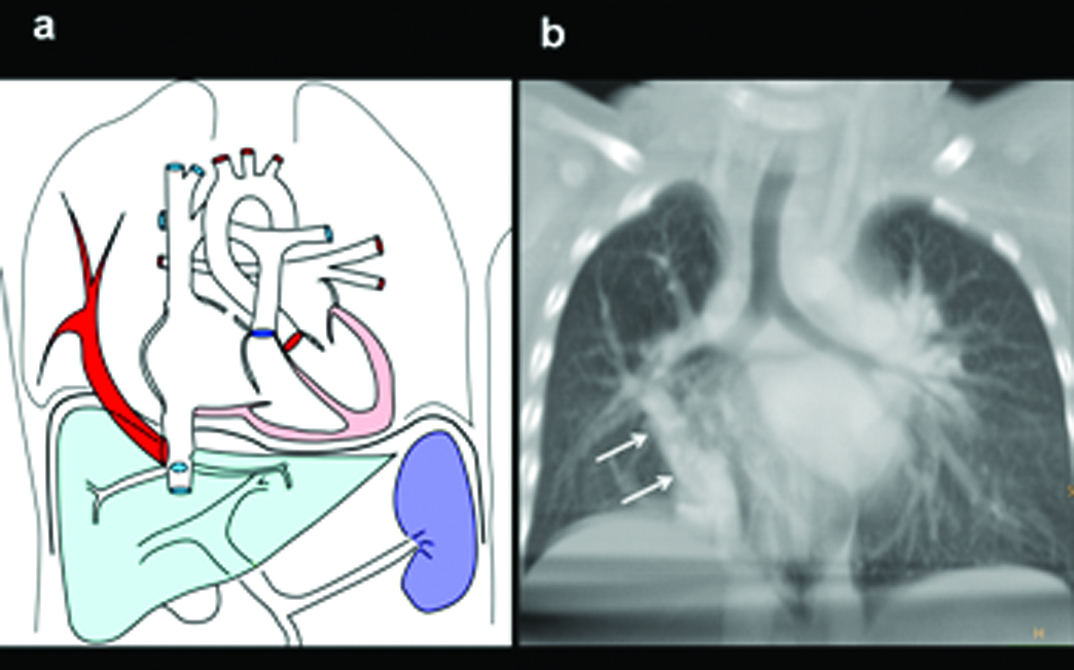
Three-year-old female presenting with a failure to thrive, left SVC on echocardiography diagnosed with partial anomalous pulmonary venous drainage of the left upper lobe: (a) Diagrammatic representation of vascular anatomy in supracardiac PAPVC. Anomalous left superior pulmonary veins is shown joining vertical vein and subsequently SVC (colour); (b) Scanogram shows moderate cardiac enlargement with increased pulmonary arterial markings and wide mediastinum (triangles); (c) axial-oblique CT images shows path of right superior pulmonary vein, vertical vein, innominate vein and SVC (arrow heads).
(d) Coronal MIP CT reconstruction shows anomalous superior pulmonary veins joining vertical vein; (e) Coronal and (f) sagittal CT image showing associated segmentation anomalies in vertebrae (arrows).
Congenital Absence of Right Pulmonary Artery
Congenital absence of pulmonary artery is a rare abnormality [Table/Fig-16a]. Most patients have associated cardiovascular anomalies like TOF, septal defects, right aortic arch and patent ductus arteriosus [8]. Clinically patients present around adolescence with recurrent pulmonary infection and haemoptysis. Collateral supply for the lung comes from systemic arteries from aorta, proximal aortic branches and occasionally form coronary arteries [Table/Fig-16b]. Right pulmonary artery is more frequently absent compared to left [8].
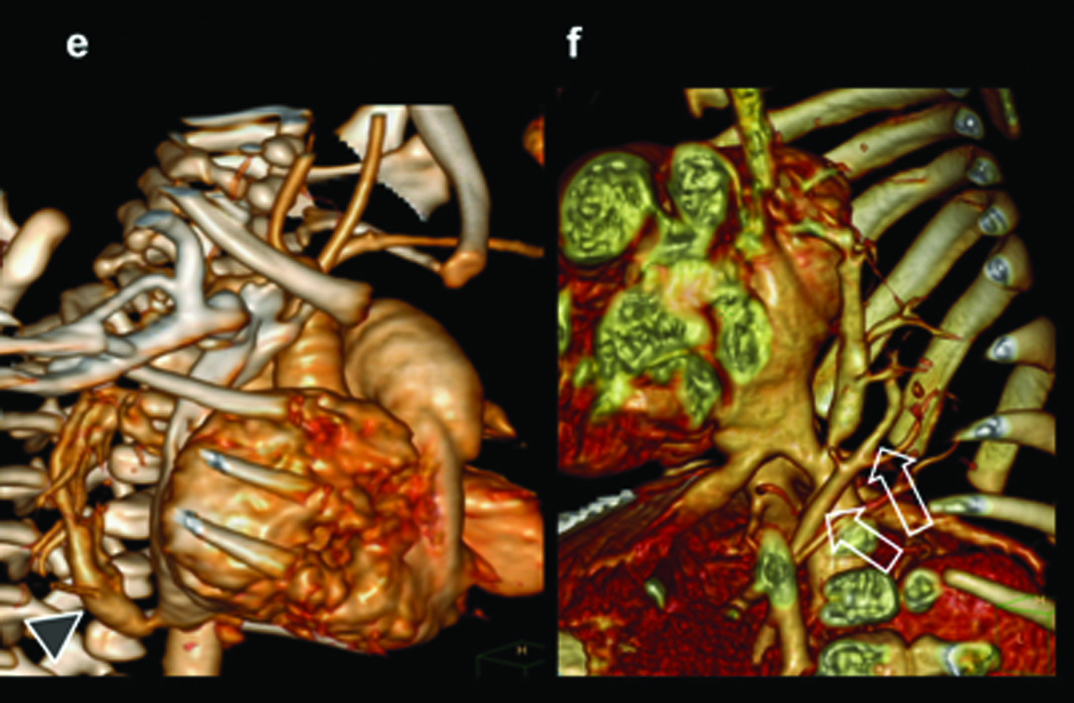
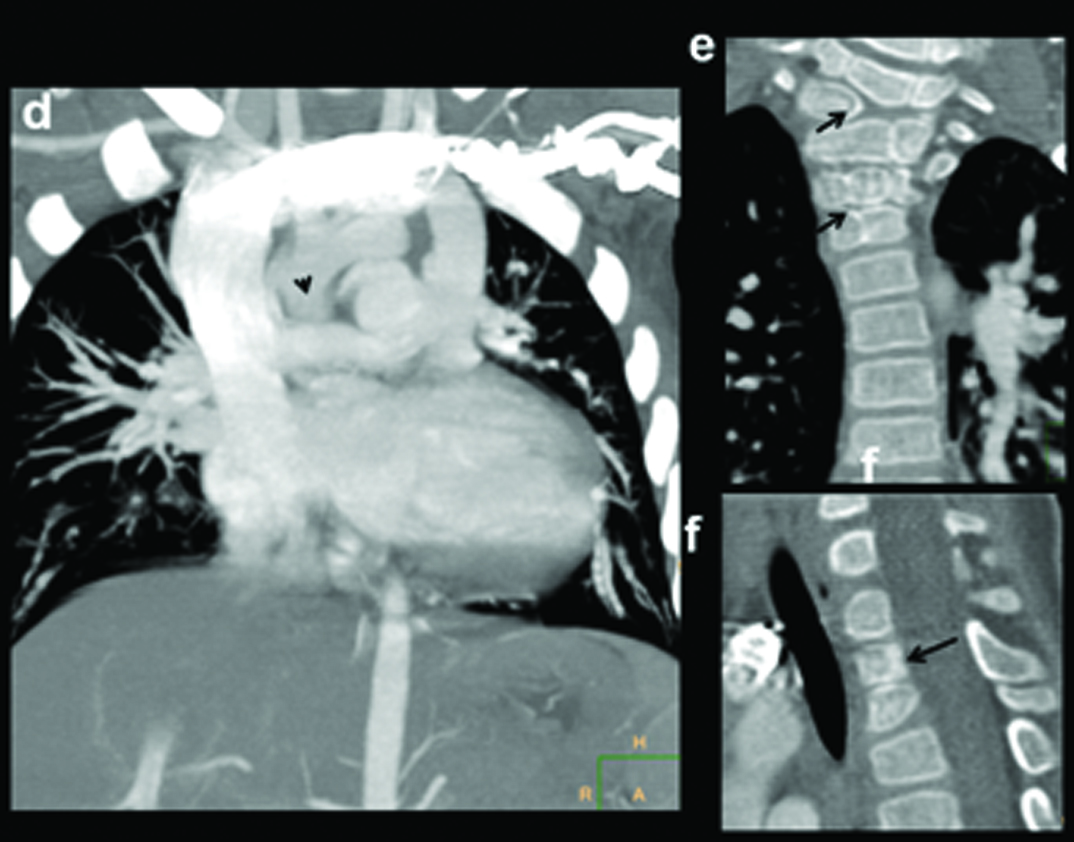
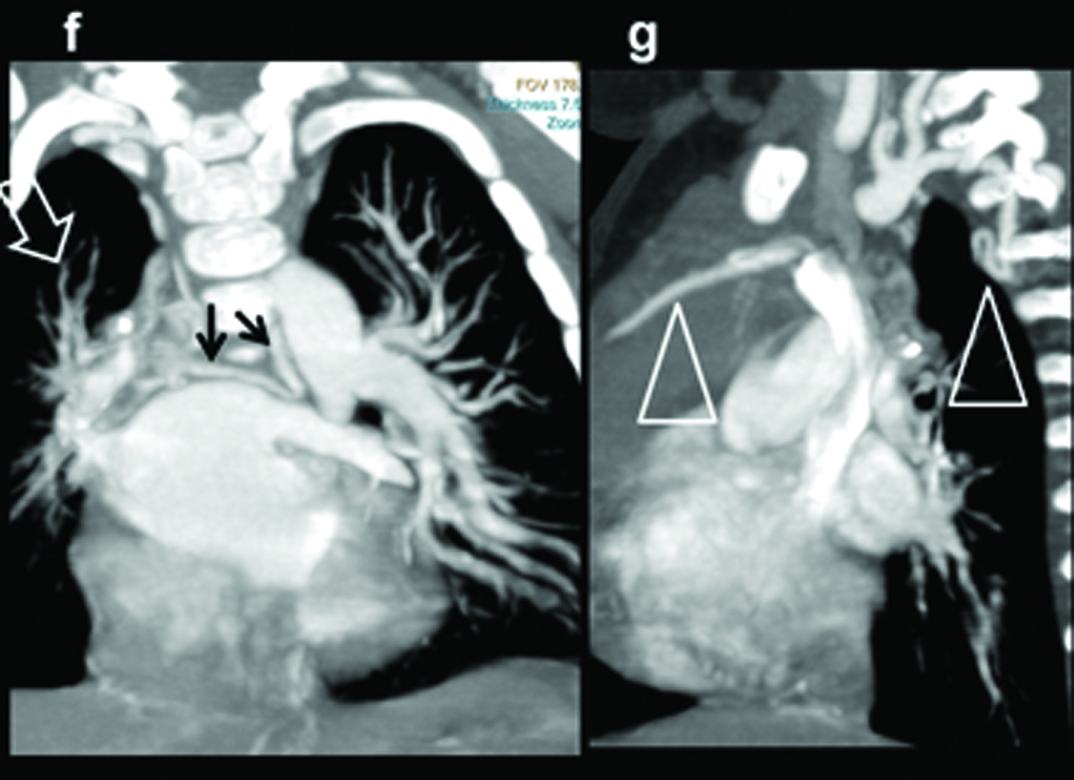
Absent right pulmonary artery. Seven-month-old male child presenting with recurrent chest infections, diagnosed with an absent right pulmonary artery: (a) Chest radiograph demonstrating the decrease in the volume of right lung and the decrease in the visualization of the pulmonary arterial branches; (b,c) axial and sagittal MIP images shows increased pulmonary arterial markings on the left side. There are thin pulmonary arterial branches on the right side; (d,e) oblique views illustrate absence of right pulmonary artery (within circle) and dilatation of the left pulmonary artery (triangle).
MIP Reconstructions in (f) coronal and (g) sagittal planes demonstrate large collaterals (black arrows, triangles) reconstituting right pulmonary artery (open arrow).
Coronary Anomalies
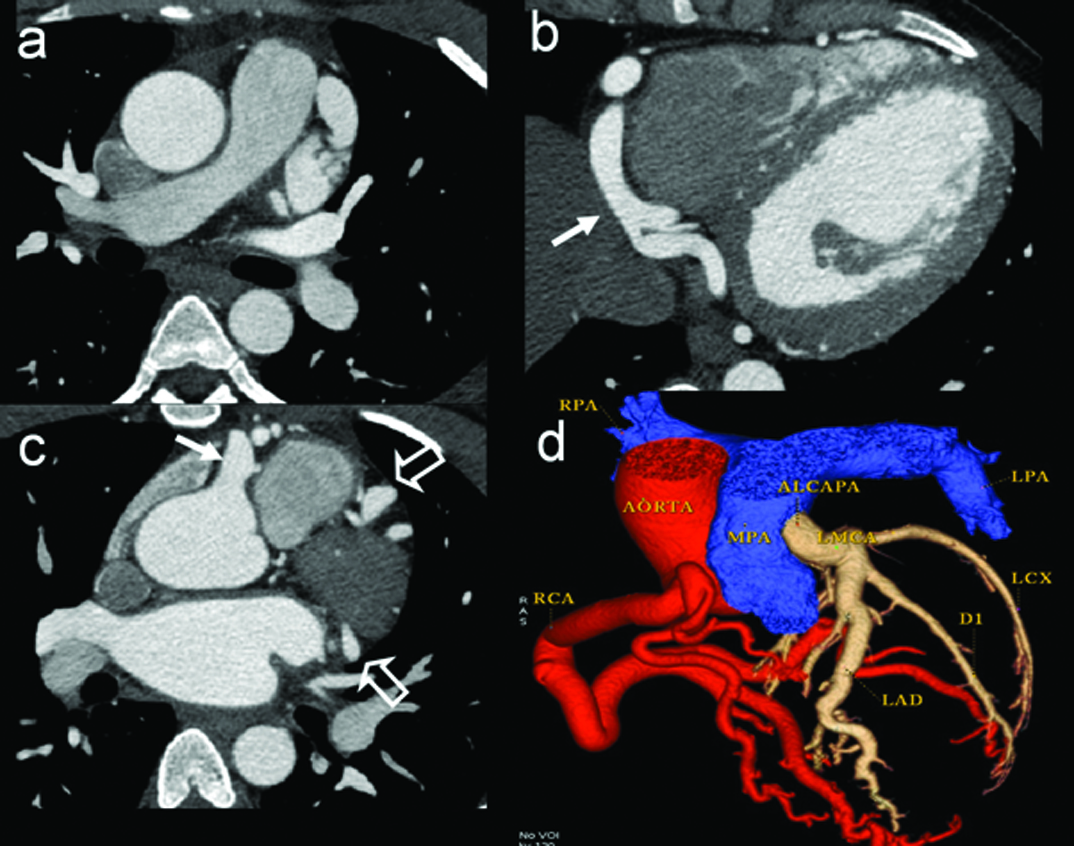
Congenital Coronary Artery Fistula (CAF) is a rare anomaly of anomalous termination of coronary arteries or branches either into a cardiac chamber, the coronary sinus, the superior vena cava, or the pulmonary artery or pulmonary vein. It is frequently demonstrated with the use of MDCT angiography, can be seen up to 1 in 250 [9]. CAFs associated with left-right shunt are clinically significant when quantity of shunt is large. Shunt leads to myocardial steal phenomenon, may culminate in myocardial ischemia, angina, infarction or arrhythmia. Proximal coronary arteries are likely site of origin of fistula. The right coronary artery (RCA) is the most commonly involved (50–55%) followed by the left anterior descending coronary artery (LAD) (35–42%) Rarely circumflex coronary artery [Table/Fig-17] or both coronary systems are simultaneously involved. Involved artery is enlarged and tortuous, may have aneurysms. Artery either drains in to a chamber or in to coronary vein. Significant numbers of patients (20–45%) with CAF have other congenital heart anomalies, such as TOF, ASD, VSD, PDA and pulmonary atresia with intact ventricular septum. Percutaneous transcatheter closure is recommended for patients with proximal fistula, fistula with a single drainage site and for surgically high risk older patients. Occlusion can be achieved by coils, umbrella devices, detachable balloons, vascular plugs, and covered stents [9]. Another interesting condition which is ideally suited for MDCT detection is a Anomalous Origin of the Left Coronary Artery (ALCAPA) [10]. In this condition left coronary artery arises from the pulmonary artery, which subsequently contribute to a left to right shunt and enlargement of the feeding the right coronary artery and the draining left coronary artery. There are infantile type and adult types [Table/Fig-18]. Infantile type leads to myocardial infarction and death due to insufficient septal collaterals. Condition is managed by surgical correction. MR imaging by virtue of its ability to assess myocardial viability can help prognosticating the need for surgical repair
Young female with a cardiac murmur suspected on echo to have a vascular anomaly: (a) Contrast axial CT demonstrate enlarged tortuous circumflex coronary artery (open arrow); (b) Axial image at a slightly higher level show circumflex arterial branches extending to SVC with luminal opacification, (white arrow) due to fistulous connection.
Seventeen-year-old male presenting with chest pain. Axial contrast CT images at three levels (a-c) demonstrate gross dilatation of right (white arrow) and left coronary artery divisions. (open arrows). Colour display of CT angiography; (d) clearly demonstrates origin of LCA from pulmonary artery (ALCAPA).
Valve and Arterial Abnormities
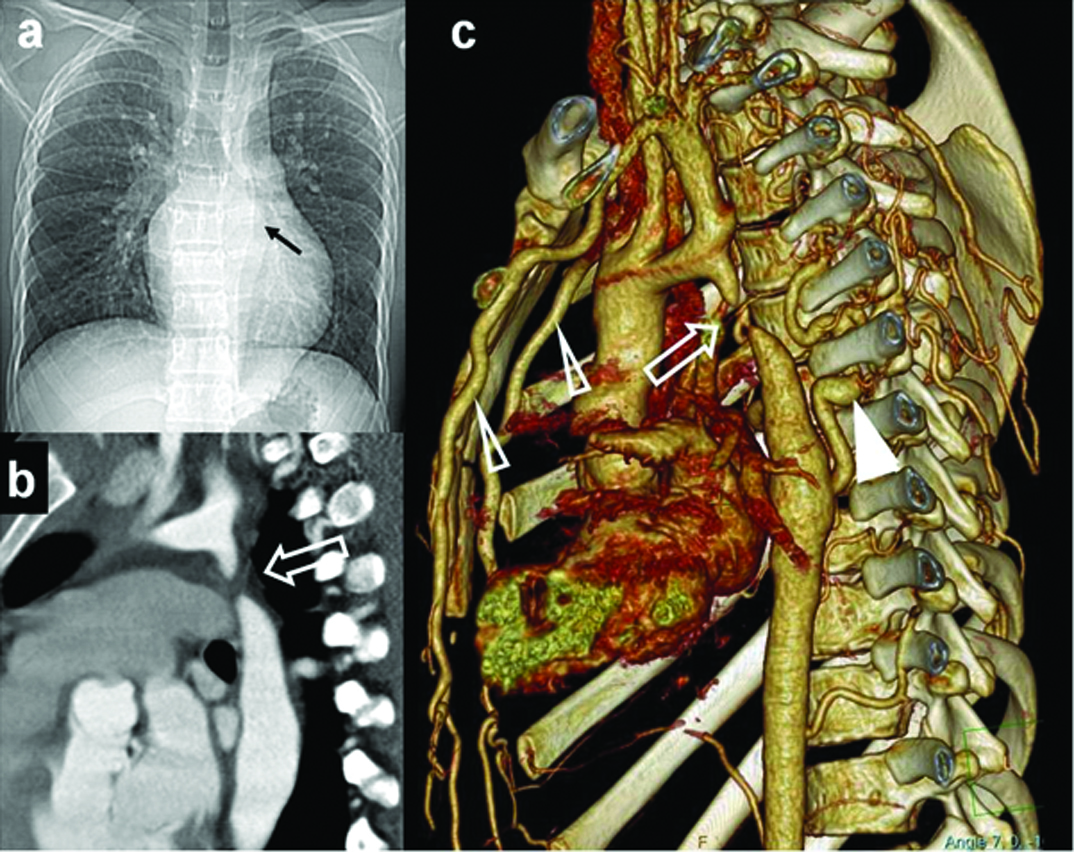
Pulmonary or aortic valves are often involved. Mild stenosis of the valve is often asymptomatic. Valvular stenosis is due to fusion of leaflets with or without annular narrowing. Diagnosis is done by echocardiography, if additional flow and functional information is needed, MR examination is performed. On plain radiography, main pulmonary artery may show dilatation. Lung vasculature is variable; either showing normal pattern or oligemia. Occasionally mitral or tricuspid valves are involved. Coarctation of aorta, though a predominant extra cardiac anomaly, often has associated cardiac lesions. Pre and post ductal varieties are described [Table/Fig-19]. CT and MR techniques are valuable to show the severity, extent of narrowing, status of collateral circulation. MR additionally contributes towards quantification of valvular flow, status of myocardial contractility and post operative follow-up.
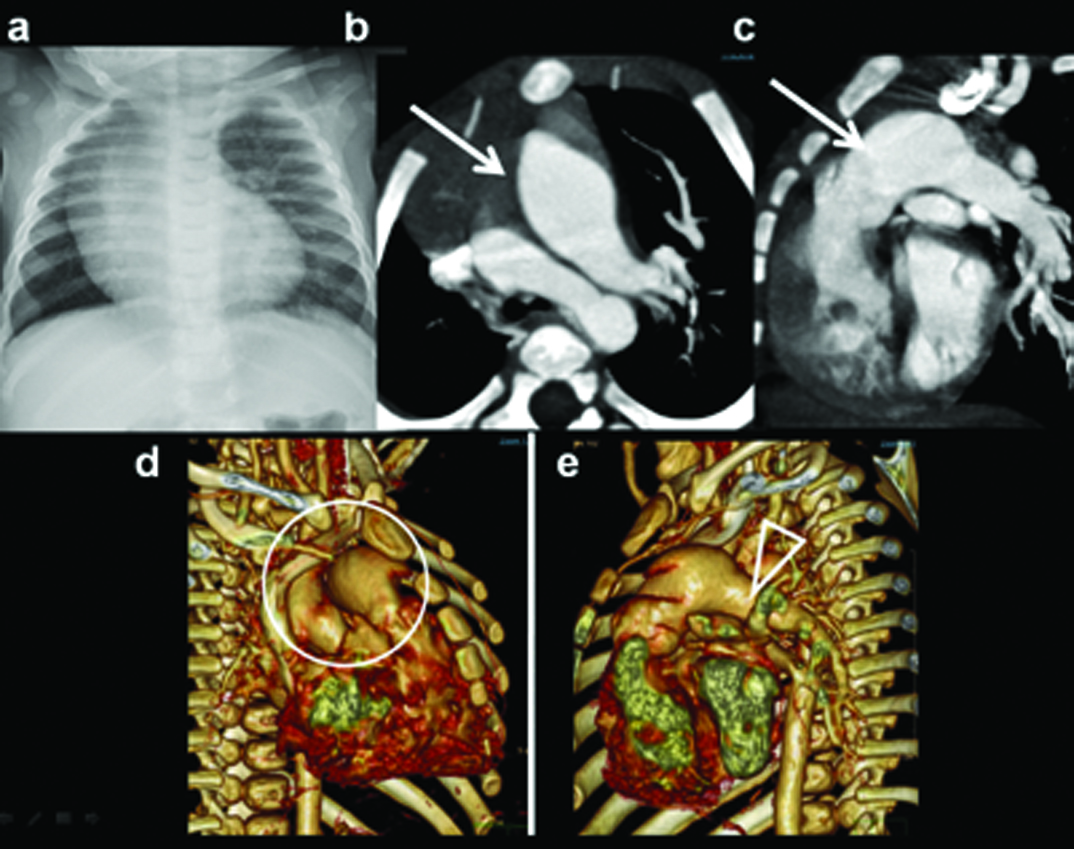
Young adult, suspected with aortic coarctation on clinical examination and echocardiography: (a) Plain radiograph shows post stenotic dilation of aorta (black arrow). Aortic knuckle is not dilated. No rib notching was noted; (b) Oblique sagittal MIP images shows the site and extent of coarctation (open arrow); (c) Volumetric 3-D rendered images illustrates site of coarctation (open arrow) and intercostal collaterals (white triangle) and dilated internal mammary arteries (open triangles).
Extracardiac Associations
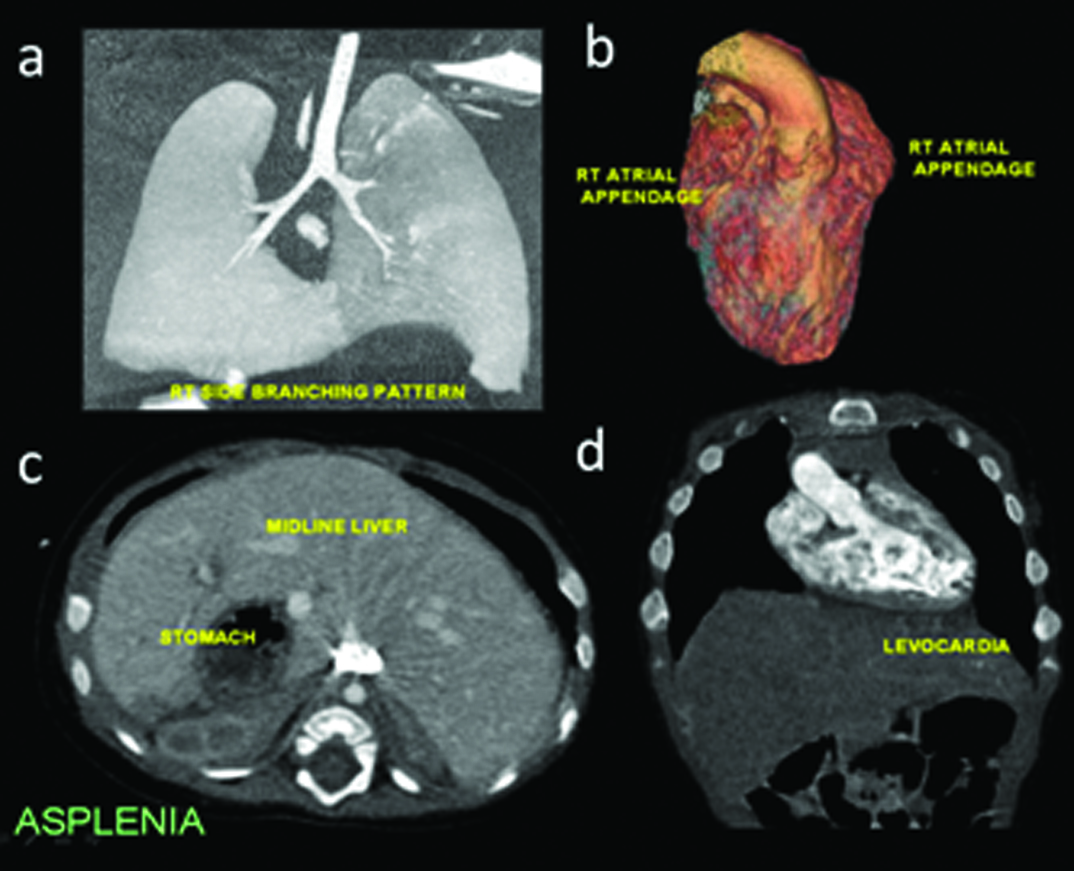
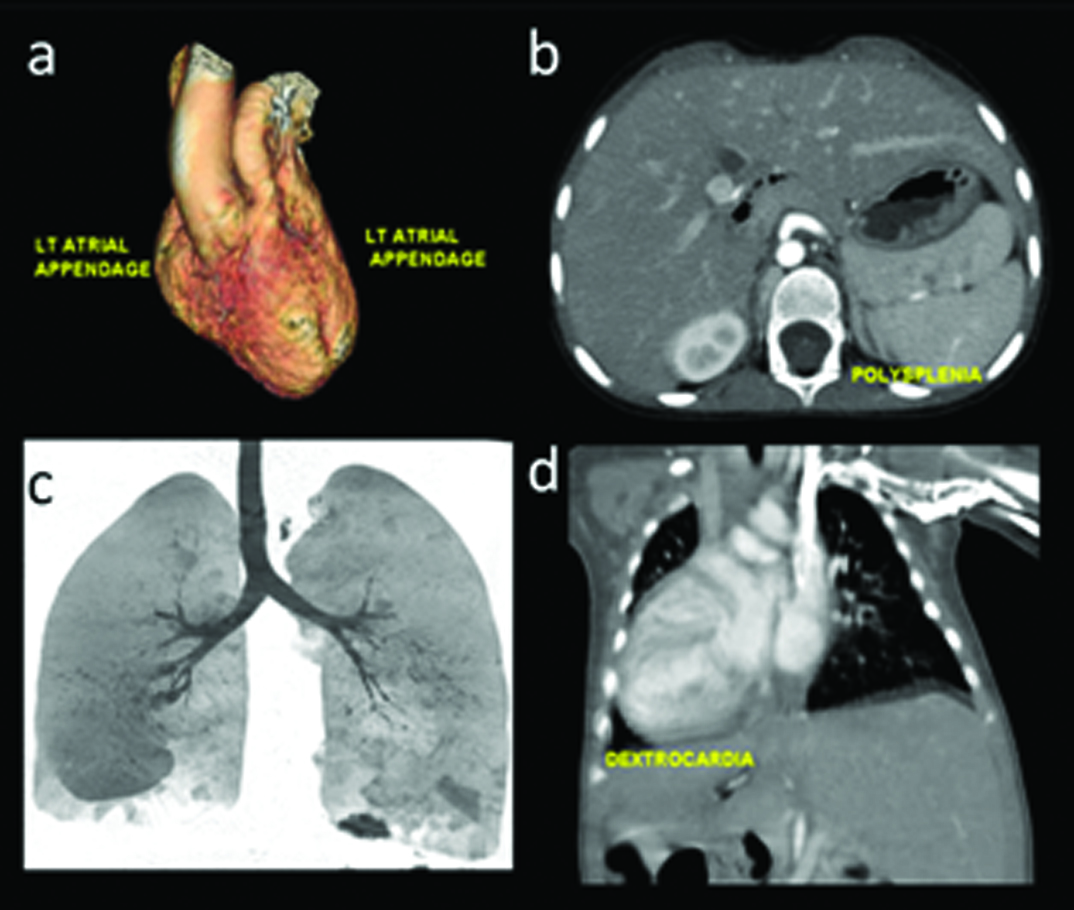
Pericardial defects are of interest which can occasionally be suspected on plain radiography due to contour deformity. MRI can contribute towards documenting abnormal myocardial contractility. Many congenital heart diseases are accompanied with abdominal visceral and vascular, especially venous malformations [11]. As described in earlier work [1], locating visceral position is an important part of establishing viscero-atrical connection. Heterotaxy syndromes with asplenia (right-sidedness [Table/Fig-20]), polysplenia (left-sidedness [Table/Fig-21]) or variable type are often noted in severe, complex cardiac anomalies. Common features in both groups is the association of atrio-venticular canal defect and intestinal malrotation. Finally pulmonary anomalies like sequestration or hypoplasia should also be scrutinised in complexes CHD, a classic example is represented by scimitar syndrome. Bony anomalies are frequent in association with CHD, an example of which is provided in [Table/Fig-15].
Heterotaxy: Asplenia features: (a) Right sided bronchial divisions; (b) bilateral right atrial appendage configuration; (c) Midline, bridged liver, situs inverses; (d) Levocardia.
Heterotaxy: Polysplenia features: (a) Bilateral left atrial appendage configuration; (b) Polyspenia; (c) Left sided bronchial divisions; (d) Dextrocardia.
Conclusion
Interpretation of imaging information of congenital heart disease is challenging, requiring knowledge of embryology, anatomy and appropriate role of imaging techniques in relation to precise clinical question. This presentation provides basic information and pathways for comprehensive analysis of some common acyanotic CHD by case illustrations. Additional examples of cardiac conditions without associated cyanosis like coronary anomalies are presented. Awareness of association of heterotaxy with congenital cardiac disease should alert the imaging specialist to look of subtle signs of visceral and venous abnormality.
[1]. Bhat V, Belaval V, Gadabanahalli K, Raj V, Shah S, Illustrated imaging essay on congenital heart diseases: multimodality approach Part I: Clinical context, anatomy and Imaging techniquesJCDR 2016 10(5):TE01-06. [Google Scholar]
[2]. O’Brien JP, Srichai MB, Hecht EM, Kim DC, Jacobs JE, Anatomy of the heart at multidetector CT: What the radiologist needs to knowRadiographics 2007 27(6):1570-82. [Google Scholar]
[3]. Goo HW, Cardiac MDCT in children: CT technology overview and interpretationRadiol Clin North Am 2011 49(5):997-1010. [Google Scholar]
[4]. Minette MS, Sahn DJ, Ventricular septal defectsCirculation 2006 114:2190-97. [Google Scholar]
[5]. Park MK, Pediatric Cardiology for Practitioners 2007 Pt IV5th edition(Ch 12)Elsevier Health Sciences:129-154. [Google Scholar]
[6]. Krichenko A, Benson LN, Burrows P, Moes CA, McLaughlin P, Freedom RM, Angiographic classification of the isolated, persistently patent ductus Arteriosus and implications for percutaneous catheter occlusionAM J Cardiol 1989 63(12):877-80. [Google Scholar]
[7]. Schneider DJ, Moore JW, Patent ductus arteriosusCirculation 2006 114:1873-82. [Google Scholar]
[8]. Mohan V, Mohan B, Tandon R, Kumbkarni S, Chhabra ST, Aslam N, Case report of isolated congenital absence of right pulmonary artery with collaterals from coronary circulationIndian Heart J 2014 66(2):220-22. [Google Scholar]
[9]. Saboo SS, Juan Y-H, Khandelwal A, George E, Steigner ML, Landzberg M, MDCT of congenital coronary artery fistulasAJR Am J Roentgenol 2014 203(3):W244-52. [Google Scholar]
[10]. Peña E, Nguyen ET, Merchant N, Dennie G, ALCAPA syndrome: not just a pediatric diseaseRadiographics 2009 29(2):553-65. [Google Scholar]
[11]. Applegate KE, Goske MJ, Pierce G, Murphy D, Situs revisited: imaging of the heterotaxy syndromeRadiographics 1999 19(4):837-52. [Google Scholar]