Coronary Artery Disease (CAD) is a major public health problem globally due to its high morbidity and mortality. CAD is a complex multifactorial disease and results from atherosclerosis of coronary arteries. Several risk factors such as modifiable (Lipids, hypertension, smoking etc.,) and non-modifiable risk factors (gender, ethnicity etc.,) are associated with CAD [1,2].
DNA damage caused due to endogenous and exogenous factors has emerged as a risk-factor for the manifestation of CAD [3,4]. A large variety of macro-vascular (structural changes in chromosomes) and micro-vascular lesions (nucleotide deletions of DNA) contribute to the development of atherosclerotic plaque in CAD [5]. Clinical trials demonstrated that statins reduce the cardiovascular complications [6].
The present study has been undertaken to assess the effect of atorvastatin therapy on lipid profiles and DNA damage in CAD patients.
Materials and Methods
The present study included 180 CAD patients from Department of Cardiology between November 2011 to December 2013 at Durgabai Deshmukh Hospital and Research Centre. Atorvastatin was administered to 180 CAD patients who had hypercholesterolaemia and no prior statin use. Blood samples were collected at index hospitalization and after 6 months statin therapy and the serum levels of lipid profiles and DNA damage were evaluated at both of those time points and also compared with 200 healthy controls. The analyses were performed with online statistical software tools.
Lipid profiles were estimated by commercially available kits and comet assay was performed as per standardized protocol.
Patient Selection and Blood Collection: Angiographically confirmed CAD patients with presumed hypercholesterolaemia and no use of statin prior to the onset of disease were enrolled prospectively. Written informed consent was obtained from the patients and healthy controls and the study was approved by the institutional ethical committee.
Patients with concomitant valvular heart disease, acute renal failures, chronic viral or bacterial infections, tumours or connective tissue diseases and those who were on dialysis were excluded from the study.
Atorvastatin is a synthetic 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase inhibitor was administered to CAD patients who were admitted in Intensive Care Unit (ICU) referred as index hospitalization. Blood samples from CAD patients were collected using EDTA-plasma collection tubes before and after 6 months on atorvastatin therapy. Two hundred healthy age and gender matched individuals were also included in the study as control subjects.
Two ml of fasting venous blood was drawn from each subject and plasma was separated by centrifugation at 3000 rpm for 15 min at 4°C and then stored at -20°C until further analysis. Lipid profiles were estimated by using commercially available kits as per manufacturer’s instructions (Erba, Transasia, Germany) and DNA damage was assessed from peripheral blood by comet assay [7]
Comet Assay: Single-Cell Gel Electrophoresis (SCGE) (comet assay)-In the present study the SCGE technique as provided by Singh et al., with alterations suggested by Ahuja and Saran was followed, except that silver staining of comets was carried out as described by Gandhi and Singh instead of using ethidium bromide [8–10].
Approximately 2 ml of venous blood was taken from each individual in a sterilised Eppendorf tube containing EDTA, and processed for assay within 2–4 hours. A 30 μl of fresh blood (around 60,000 cells) mixed with 130 μl of 0.7% Low-Melting Point Agarose (LMPA) in phosphate buffered saline at 37°C was layered onto a slide that was pre-coated with thin layers of 1% normal melting point agarose. The slides were left for five minutes at 4°C in order to allow the agarose to solidify, and this was followed by another layer of LMPA. The slides were then subjected to the lytic process in freshly prepared cold (4°C) lysing solution (2.5 M NaCl, 100 mM EDTA-2Na, 10 mM Tris-HCl, pH 10–10.5, 1% Triton X-100 and 10% dimethyl sulfoxide added just before use) for at least 8–12 hours and then immersed in freshly prepared alkaline electrophoresis buffer (0.3 mol/l NaOH, and 1 mmol/l Na2EDTA, pH > 13) at 4°C for unwinding (40 minutes), followed by electrophoresis (25 V/300 mA, 25 minutes). All the steps were carried out under minimal illumination and stained with silver nitrate (AgNO3) solution. For each sample, the slides were prepared in duplicate, and a total of 100 cells were scored under binocular microscope at 40 times magnification using an oculometer. DNA damage was calculated as the difference between the length of the comet and the diameter of the comet head.
Statistical Analysis
The analysis were performed with online statistical software tools such as Open Epi version 3.03 to examine the differences in demographic and clinical variables between CAD patients before and after statin therapy and healthy controls. We used ANOVA for calculating p-value and a p-value ≤0.05 was used as the criterion of statistical significance. All numeric values are expressed as the mean ± SD.
Results
Baseline characteristics of the CAD patients and controls are shown in [Table/Fig-1]. Age, gender, and risk-factor profiles (SBP, DBP, Smokers) significantly differed between the CAD patients and healthy control groups (p≤0.01). The changes in the lipid profiles and DNA damage (Comet tail length) before and after statin treatment in CAD patients as well as healthy controls are shown in [Table/Fig-2,3].
Demographic and clinical data in the controls and patients.
| Sl No | Variables | Controls(n=200)mean±SD | CAD patients(n=180)mean±SD | p-value |
|---|
| 1 | Female/Male | 85/115 | 60/120 | 0.066 |
| 2 | Age (yrs) | 54.55±14.39 | 55.22±13.61 | 0.44 |
| 3 | BMI | 23.8±3.27 | 24.96±3.05 | 0.341 |
| 4 | SBP(mmHg) | 120.23±5.86 | 140±22.2 | <0.05* |
| 5 | DBP(mmHg) | 80.89±3.22 | 82.9±9.91 | <0.05* |
| 6 | Smokers/ Non-smokers | 45/155 | 100/80 | <0.05* |
| 7 | Family History | 0 | 78/102 | – |
Data represented as mean ± standard deviation and* SBP, DBP, Smoking parameters are statistically significant (p < 0.05).
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI; Body Mass Index
Lipid profiles Controls, CAD patients ((ICU), their 6 months follow-ups on statin therapy.
| Sl No | Variable | Controls(n=200) | CAD patients(ICU) (n=180) | CAD patientsafter 6 months(n=135) | p-value |
|---|
| 1 | Total Cholesterol (mg/dL) | 140.14±26.51 | 230.72±54.36 | 159.6±48.8 | <0.05** |
| 2 | Triglyceride (mg/dL) | 133.24±29.31 | 177.46±87.9 | 148±75.3 | <0.05** |
| 3 | LDL (mg/dL) | 69.69±27.15 | 161.48±64.1 | 134.78±15.16 | <0.05** |
| 4 | HDL (mg/dL) | 43.77±12.90 | 31±14.4 | 34±15.37 | 0.073 |
** Data represented as mean ± standard deviation and Total Cholesterol, Triglycerides, LDL were statistically significant at p<0.05
Comet tail lengths of Controls and CAD patients, their 6 months follow-ups on statin therapy.
| Parameter(Mean ± SD) | Controlsn=200 | Intensive CareUnit –CAD Patientsn=180 | Statin therapy(6 months)n=135 | p-value |
|---|
| Comet tail length(μm) | 10.2 ± 3.25 | *18.89 ± 5.78 | *14.2 ± 3.32 | <0.05* |
*Statistically significant (p <0.05).
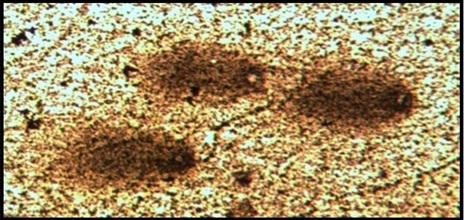
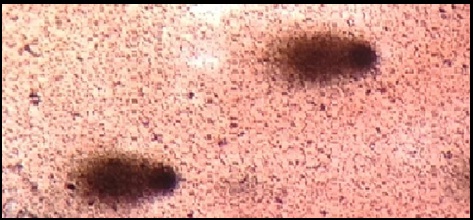
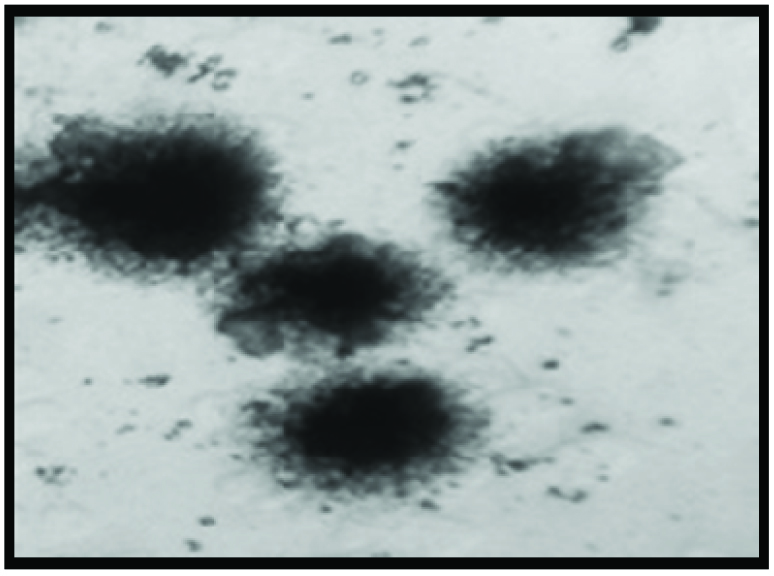
The DNA damage was significantly high in CAD patients before atorvastatin treatment and is reduced after 6 months atorvastatin therapy as shown in [Table/Fig-4,5]. While healthy controls did not showed DNA damage compared to CAD patients as shown in [Table/Fig-6].
Comet cell before atorvastatin treatment (ICU).
Comet cells after atorvastatin treatment (after 6 months).
Normal cells in healthy controls.
Discussion
CAD is a consequence of atherosclerosis of coronary arteries. Interplay between multiple genes and environmental factors and increased atherogenic oxidized LDL causes cytotoxicity and DNA damage in cells of arterial wall, leading to progression of cell senescence, cell death of an atherosclerotic plaque [11]. There is a paucity of data regarding the changes in DNA damage in CAD patients before and after atorvastatin use.
ROS (Reactive Oxygen Species) are involved in the pathogenesis of atherosclerosis and might play critical roles in DNA damage. Thus, measurement of DNA damage in peripheral blood provides a useful tool for exploring the pathophysiological mechanisms and assessing the effects of atorvastatin on CAD patients.
Statins are the most commonly prescribed cholesterol lowering drugs used in the treatment of cardiovascular diseases. Statins not only lowers the cholesterol, but also have pleiotropic effects, including reduction of inflammation, improvement of endothelial function, antioxidant and anti-inflammatory effects etc., [12].
Mahmoudi et al., summarized that the statins reduce the degree of DNA damage and oxidant stress in hyperlipidemic patients [5]. Mondal et al., found that elevated oxidative stress and DNA damage in heart failure patients [13].
LaRosa et al., concluded that atorvastatin has a significant clinical benefit on lipid profiles of CAD patients [14]. Mariann H et al., findings showed that atorvastatin treatment decreases the DNA damage and lipid profile [15].
In another study Sedon et al., discussed that the use of statins inhibits the activation of small GTPases such as Rac, thereby affecting NADPH oxidase activity which decreases DNA Damage [16]. The present study is also consistent with previous reports where usage of atorvastatin decreases the DNA damage in CAD patients. However further studies are needed to establish the effects of atorvastatin on DNA damage after long-term statin use.
Limitation
Our study has some limitations. This study was a relatively small cohort, conducted in a single center. The study included homogenous groups of confirmed CAD patients on atorvastatin therapy. These results represent the short term effects of atorvastatin therapy and long-term follow-up data are needed. Further detailed investigations from similar population are necessary to overcome these limitations.
Conclusion
The results of this study suggests the need for a comprehensive approach and short-term use of a high-dose statin (80mg) has not only lipid lowering but also prevents DNA damage in CAD patients. Further studies are also needed to establish the long-term effects of atorvastatins in CAD patients. To the best of our knowledge this is the first study reporting on therapeutic effect of atorvastatin on lipid profiles and DNA damage in south Indian population.
Data represented as mean ± standard deviation and* SBP, DBP, Smoking parameters are statistically significant (p < 0.05).
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI; Body Mass Index
** Data represented as mean ± standard deviation and Total Cholesterol, Triglycerides, LDL were statistically significant at p<0.05
*Statistically significant (p <0.05).