Buccal Micronucleus Cytome Assay in Sickle Cell Disease
Mallika Bokka Sri Satya Naga1, Shreya Gour2, Nalini Nallagutta3, Kranti Kiran Reddy Ealla4, Surekha Velidandla5, Sangameshwar Manikya6
1 Postgraduate Student, Department of Oral and Maxillofacial Pathology, MNR Dental College & Hospital, Sangareddy, Telangana, India.
2 Postgraduate Student, Department of Oral and Maxillofacial Pathology, MNR Dental College & Hospital, Sangareddy, Telangana, India.
3 Postgraduate Student, Department of Oral and Maxillofacial Pathology, MNR Dental College & Hospital, Sangareddy, Telangana, India.
4 Reader, Department of Oral and Maxillofacial Pathology, MNR Dental College & Hospital, Sangareddy, Telangana, India.
5 Reader, Department of Oral and Maxillofacial Pathology, MNR Dental College & Hospital, Sangareddy, Telangana, India.
6 Senior Lecturer, Department of Oral and Maxillofacial Pathology, MNR Dental College & Hospital, Sangareddy, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kranti Kiran Reddy Ealla, Reader, Department of Oral and Maxillofacial Pathology, MNR Dental College & Hospital, Fasalwadi, Sangareddy, Telangana State-502294, India.
E-mail: drekkr@yahoo.co.in
Introduction
Sickle Cell Anaemia (SCA) is a commonly inherited blood disorder preceded by episodes of pain, chronic haemolytic anaemia and severe infections. The underlying phenomenon which causes this disease is the point mutation in the haemoglobin beta gene (Hbβ) found on chromosome 11 p. Increased oxidative stress leads to DNA damage. DNA damage occurring in such conditions can be studied by the buccal micronucleus cytome assay, which is a minimally invasive method for studying chromosomal instability, cell death and regenerative potential of human buccal tissue.
Aim
To evaluate genomic instability in patients with sickle cell disease by buccal micronucleus cytome assay.
Materials and Methods
The study included 40 sickle cell anemia patients (Group A) and 40 age and sex matched controls (Group B). Buccal swabs were collected and stained with Papanicolaou (PAP). Number of cells with micronucleus, binuclei, nuclear bud, pyknosis and karyolysis were counted in two groups as parameters for the evaluation of genome stability.
Results
All the analysis was done using t-test. A p-value of <0.001 was considered statistically significant. There was a statistically significant increase in micronuclei number in SCA patients when compared with controls. Karyolytic (un-nucleated) cell number in Group A was more than to those of the controls.
Conclusion
The results might suggest that patients with sickle cell anaemia have genome instability which is represented by the presence of micronuclei in the somatic cells. Presence of apoptotic cells might only indicate the bodily damage to the tissue as a result of the disease.
Binuclei, Karyolysis, Nuclear bud, Pyknosis
Introduction
In Sickle Cell Anaemia (SCA), the point mutation in the haemoglobin beta gene (Hbβ) found on chromosome 11 p, leads to formation of structurally abnormal haemoglobin, called Hbs [1–3]. Under certain conditions such as low oxygen level or high haemoglobin concentration, in individuals who are homozygous for Hbs, the abnormal Hbs cluster together which then deform the RBCs into sickle shapes. These sickled RBCs become trapped in small blood vessels and block them, producing pain and eventually damaging organs. This causes hand-foot syndrome, fatigue, paleness and shortness of breath, pain that occurs in any body organ or joint. Patients also suffer from yellowish skin and eyes and delayed growth and puberty in children. Those individuals who possess one copy of the normal Hbβ gene and one copy of the sickle variant (Hbs) are referred to as having sickle cell trait, but these individuals do not express symptoms of sickle cell disease [4].
A MicroNucleus (MN) is formed during the metaphase/anaphase transition of mitosis. It may rise from a whole lagging chromosome {a eugenic after breakage (Clastogenic event) which do not integrate in the daughter nuclei}. The buccal cell MicroNucleus (MN) assay, first proposed by Stich et al, is useful as a biomarker of genetic damage. It is caused by life-style habits (e.g., smoking consumption and/or alcohol micronutrient deficiency) and exposure to environmental pollutants as well as in patients with inherited genetic defects in DNA repair [5,6]. This study might help in monitoring the effects of therapeutic agents used to treat the disease and thus improvise the treatment modalities.
Aim
The study aim was to assay the genomes’ stability in patients with sickle cell anaemia by a micronucleus assay in buccal mucosa cells.
Materials and Methods
Study population: It was a cross-sectional observational study and was carried on saliva samples of SCA patients taken from Thalassemia and Sickle Cell Society, Hyderabad, India. Thalassemia and Sickle Cell Society of Hyderabad (TSCS), founded in 1998, works in creating awareness about SCA among the people. Parents and extended family of the child get genetic counselling and primary care is provided in management and prevention of these genetic disorders. The society promotes new research in this disease and permission was granted to carry out the study after the project was presented for approval. The study included 40 SCA patients and 40 age and sex matched controls. Informed consent was taken from the patients prior to the study. The study was carried out in the Department of Oral and Maxillofacial Pathology, MNR Dental College and Hospital, Hyderabad, India. Approval from the institutional ethical committee board was taken before pursuing the research study.
Patient selection was based on following inclusion and exclusion criteria.
Inclusion criteria
Patients previously diagnosed with SCA.
Fully conscious and cooperative patients with good oral hygiene.
Exclusion criteria
Patients with severe jaundice and hemolytic anemia were excluded.
Patients indulged in chronic smoking and alcoholics.
Children who utilized mouthwashes or orthodontic braces as well as presenting lesions in their oral mucosa.
Patients with findings of any physical or mental abnormality, which would interfere with or be affected by the study procedure.
Cell sampling and preparation: Forty individuals of the same age group and gender volunteered for the study and were included in the control group.
Buccal mucosa cells (BC) were obtained from the consented volunteers by following the method of Tolbert et al., and Belien et al., [7,8]. The mouth of each volunteer was rinsed thoroughly with water to remove any unwanted debris before BC collection. Small-cotton headed sterilized sticks were rotated several times in a circular motion against the inner mucosal lining of the cheek. The head of the stick (cotton swab) was swapped on clean glass slides to make smear. Slides were then fixed in ethyl alcohol for 10 minutes, rinsed with distilled water and stained with PAP for 20 minutes and air dried.
Scoring method: The best possible method to score slides in this cytological assay is to first determine the frequency of all the various cell types in a minimum of 1,000 cells [8]. Following this step, the frequency of DNA damage biomarkers is scored in a minimum of 2,000 differentiated cells.
Results
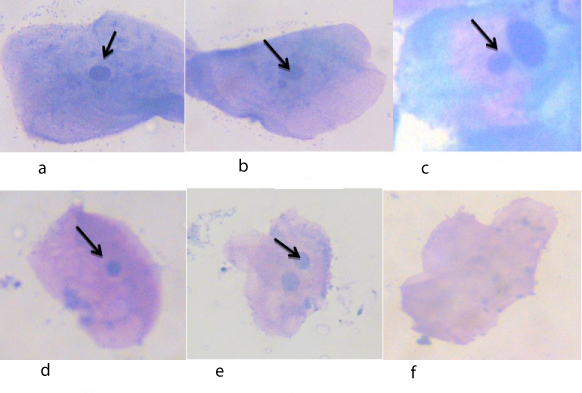
An Independent t-test was computed to analyse the mean number of micronuclei, binuclei, pyknosis, karyolysis among healthy and SCA patients. The results for cells with MNs, binucleated cells, karyolytic cells and pyknotic cells are summarized and illustrated in [Table/Fig-1,2]. The cells with MNs, binucleated cells, karyolytic cells and pyknotic cells were visible as in [Table/Fig-3a-f]. Cells with micronuclei were significantly (p<0.001) more in patients with SCA group (MN 2-12/1000cells), compared to controls (0.65MN/1000 cells) with a range of 1-4 cells/1000 cells. karyolytic cells, binuclear cells, pyknotic cells were significantly higher in number in patients with SCA. Other nuclear abnormalities such as binucleus, pyknosis and karyolysis were also increased in sickle cell anaemia patients when compared with healthy controls and the results were statistically significant (p< 0.001).
The frequencies of micronuclei and other nuclear abnormalities in Group A.
| Nuclear Abnormalities | Number | Minimum | Maximum | Mean | Std. Deviation |
|---|
| Micronuclei | 40 | 5.00 | 13.00 | 9.2250 | 2.05673 |
| Binuclei | 40 | 0.00 | 2.00 | 0.4750 | 0.67889 |
| Pyknosis | 40 | 0.00 | 2.00 | 0.5750 | 0.67511 |
| Karyolysis | 40 | 0.00 | 2.00 | 0.6500 | 0.69982 |
The frequencies of micronuclei and other nuclear abnormalities in Group B.
| Nuclear Abnormalities | Number | Minimum | Maximum | Mean | Std. Deviation |
|---|
| Micronuclei | 40 | 0.00 | 4.00 | 1.8000 | 1.39963 |
| Binuclei | 40 | 0.00 | 0.00 | 0.0000 | 0.00000 |
| Pyknosis | 40 | 0.00 | 0.00 | 0.0000 | 0.00000 |
| Karyolysis | 40 | 0.00 | 0.00 | 0.0000 | 0.00000 |
Image showing (a) Normal buccal cell, (b) & (c) Buccal cell with micronuclei, (d) Pyknotic cell, (e) Binucleated cell, (f) Karyolytic cell.
Discussion
Buccal epithelial cells are a recognized target site for early genotoxic events induced by carcinogenic substances. Thus, the application of micronucleus test in buccal epithelium cells has been increasingly accepted as a sensitive tool for bio-monitoring the genetic damage in human population [9]. We studied the MNs frequency in buccal cells in SCA patients and controls to understand the role of the different biomarkers and their relationship with the extremely variable clinical manifestation of sickle cell disease.
The current study showed an increase in micronuclei and nuclear anomalies like binuclei, nuclear bud, pyknosis and karyolysis in SCA patients when compared with controls. However, our study showed a statistically significant increase in micronuclei in SCA when compared with controls. Our study is in accordance with previous studies done by E K. Shubber et al., and Mishra N et al., [10,11]. They also demonstrated high micronuclei frequency in SCA when compared with controls.
Causes of the presence of micronuclei in buccal cells from SCA patients are unknown. However, Klings ES and Alves et al., had reported that SCA patients are subjected to increased oxidative stress particularly during vaso-occlusive crises and acute chest pain [12,13]. This results in chromosomal breakage and subsequently leads to the formation of micronuclei. Another possible cause of oxidative stress in SCD is the high concentration of iron in the patients’ plasma. The increase in oxidative stress could be a relevant risk factor for mutagenesis and carcinogenesis.
Folic acid and vitamin B12 depletion in SCA patients also causes occurrence of micronuclei. Folic acid and B12 play a critical role in the prevention of chromosome breakage that leads to micronucleus formation. A deficiency of folic acid and other such nutrients may result in the gross change of genomic integrity and alteration of DNA methylation, thus linking nutrition with the modulation of gene expression [14]. B N Ames demonstrated that decrease in folic acid causes chromosomal breakage in human genes [15]. The result is an increase in the cellular dUMP/dTMP ratio and DNA polymerase-mediated deoxyuridine triphosphate misincorporation into genomic DNA. Loss of uracil from DNA generates single-strand breaks that could result in a double-strand break if two opposing nicks are formed. [16]. Various studies, both in vitro and in vivo, with human cells clearly show that folate B12 deficiency causes expression of chromosomal fragile sites, chromosome breaks, excessive uracil in DNA, micronucleus formation and DNA hypermethylation [17,18]. Micronucleus observed in buccal cells is not induced when the cells are at the epithelial surface but are formed in the basal layers.
Karyolytic cells represent buccal cells undergoing apoptosis. These cells act as a biomarker in clinical trials of cancer therapy as well as in patients with Alzheimer’s disease who have higher frequency of MN in their buccal cells [19]. The results of our study showed that patients with SCA have elevated levels of un-nucleated cells compared to those of the control. In association with karyolytic cells, there was increase in pyknosis and binuclei in patients with SCA when compared with healthy controls. This result might suggest that patients with SCA have genome instability which is represented by the presence of micronuclei in the somatic cells [20].
The MN assay is regarded as an important biomarker to predict the relative risk of cancer. Thus, greater prevalence of MN in SCA patients implies that they are highly exposed to mutagenic insults and therefore, are at a greater risk of developing cancer. The present study revealed the presence of a high genotoxic risk in patients with SCA.
Conclusion
Our study revealed a significant increase in MN count in sickle cell anaemia when compared with controls. There was also increase in binuclei, pyknosis and karyolysis in sickle cell anaemia patients when compared with controls. This suggests the presence and increase of the various forms of cells in SCA when compared to controls. The nuclear abnormalities evident in buccal mucosa cells may help in effective monitoring and treatment outcomes in patients with SCA.
[1]. Serjeant GR, Sickle Cell Disease 1988 OxfordOxford medical Publicaltion:7 [Google Scholar]
[2]. Fleming AF, Sickle cell disease 1982 58EdinburghChurchill Livingstone:6 [Google Scholar]
[3]. Vichinsky EP, Lutoin BH, Sickle cell anemia and related hemoglobinopathiesPediatr Clin North Am 1980 2:247-49. [Google Scholar]
[4]. Dash S, Haemoglobin S-D disease in a Bahraini childBahrain Med Bull 1994 17:154-55. [Google Scholar]
[5]. Stich HF, Rosin MP, Use of the micronucleus test to monitor the effect of vitamin A, β-carotene and canthoxanthin on the buccal mucosa of betel nut /tobacco chewersInt J Cancer 1984 34:745-50. [Google Scholar]
[6]. Chen C, Arjomandi M, Quin H, Balmes J, Tager I, Holland N, Cytogenetic damage in buccal epithelial and peripheral lymphocytes of young healthy individuals exposed to ozoneMutagenesis 2006 21:131-37. [Google Scholar]
[7]. Tolbert PE, Shy CM, Allen JW, Micronucleus and other nuclear anomalies in buccal smears, methods developmentMutation 1992 :669-77. [Google Scholar]
[8]. Belien JA, Copper MP, Braakhuis BJ, Snow GB, Baak JP, Standardization of counting micronuclei: definition of a protocol to measure genotoxic damage in human exfoliated cellsCarcinogensis 1995 16(10):2395-400. [Google Scholar]
[9]. Sagari SG, Babannavar R, Lohra A, Kodgi A, Bapure S, Rao Y, Micronuclei frequencies and nuclear abnormalities in oral exfoliated cells of nuclear power plant workersJ Clin Diagn Res 2014 8(12):ZC15-17. [Google Scholar]
[10]. Shubber EK, AL-Julandi JK, AL-Rawahi SK, Micronucleus frequencies in buccal cells from patients with sickle cell anaemiaIraqi Journal of Cancer and Medical Genetics 2010 3(1):28-32. [Google Scholar]
[11]. Mishra N, Kumar A, Assessment of micronuclei frequency in sickled tribal population (Halba and Gond) of district Durg, Chhattisgarh, IndiaAdv Bio Res 2014 5(4):125-30. [Google Scholar]
[12]. Klings ES, Farber HW, Role of free radicals in the pathogenesis of acute chest syndrome in sickle cell diseaseRespir Res 2001 2:280-85. [Google Scholar]
[13]. Alves PM, Martins PR, Dias Fda L, Burbano RM, Bianchi Mde L, Antunes LM, Sensitivity to cisplatin-induced mutations and elevated chromosomal aberrations in lymphocyte from sickle cell disease patientsClin Exp Med 2008 8:31-35. [Google Scholar]
[14]. Arigony ALV, Oliveira IMD, Machado M, Bordin DL, Bergter L, Prá D, The influence of micronutrients in cell culture: a reflection on viability and genomic stabilityBio Med Research International 2013 :1-22. [Google Scholar]
[15]. Ames BN, DNA damage from micronutrient deficiencies is likely to be a major cause of cancerMutat Res 2001 475(1-2):7-20. [Google Scholar]
[16]. Ashutosh Lal, Bruce N. Ames, Association of chromosome damage detected as micronuclei with hematological diseases and micronutrient statusMutagenesis 2011 26(1):57-62. [Google Scholar]
[17]. Crott JW, Mashiyama ST, Ames BN, Fenech M, The effect of folic acid deficiency and MTHFR C677T polymorphism on chromosome damage in human lymphocytes in vitroCancer Epidemiol Biomarkers Prev 2001 10:1089-96. [Google Scholar]
[18]. Fench M, The role of folic acid and vitamin B12 in genomic stability of human cellsMutat Res 2001 475:57-67. [Google Scholar]
[19]. Fench M, Chang WP, Kirsch–Volders M, Holland N, Bonassi S, Zeiger E, Human project: detailed description of the scoring criteria for the cytokinesis –block micronucleus assay using isolated human lymphocyte culturesMutat Res 2003 534:65-75. [Google Scholar]
[20]. Bloching M, Hofmann A, Lautenschlager C, Berghaus A, Grummt T, Exfoliative cytology of normal buccal mucosa to predict the relative risk of cancer in the upper aerodigestive tract using the MN assayOral Oncol 2000 36:550-55. [Google Scholar]