Biofilm in Osteomyelitis caused by a Rare Pathogen, Morganella morganii : A Case Report
Asmita De1, Hirak Jyoti Raj2, Prasanta Kumar Maiti3
1 Post Graduate Student, Department of Microbiology, Institute of Post-Graduate Medical Education and Research, Kolkata, India.
2 Assistant Professor, Department of Microbiology, Institute of Post-Graduate Medical Education and Research, Kolkata, India.
3 Professor and Head, Department of Microbiology, Institute of Post-Graduate Medical Education and Research, Kolkata, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Hirak Jyoti Raj, J-5, Sahapur Govt. Housing Estate, New Alipore, Kolkata-700038, India.
E-mail: hirak17raj@gmail.com
Morganella morganii is a member of Enterobacteriaceae family, whose natural habitat is the human gastrointestinal tract. It rarely causes infection alone and is generally encountered in immunosuppressed patients. Osteoarticular pathologies are not commonly observed with Morganella morganii and infections by it have high mortality rate. Biofilm colonization is a causative factor behind the chronicity and/or refractoriness of certain infections. Biofilms colonize on inert medical devices, prosthesis, fibrosed tissues, sinus tracts as well as dead bones as in case of chronic osteomyelitis. Morganella morganii is not a common pathogen to produce biofilm. In this case report, we present a 56-year-old male patient with chronic osteomyelitis of right proximal tibia caused by biofilm producing strain of Morganella morganii, following trauma.
Chronic osteomyelitis, Immunosuppression, Osteoarticular
Case Report
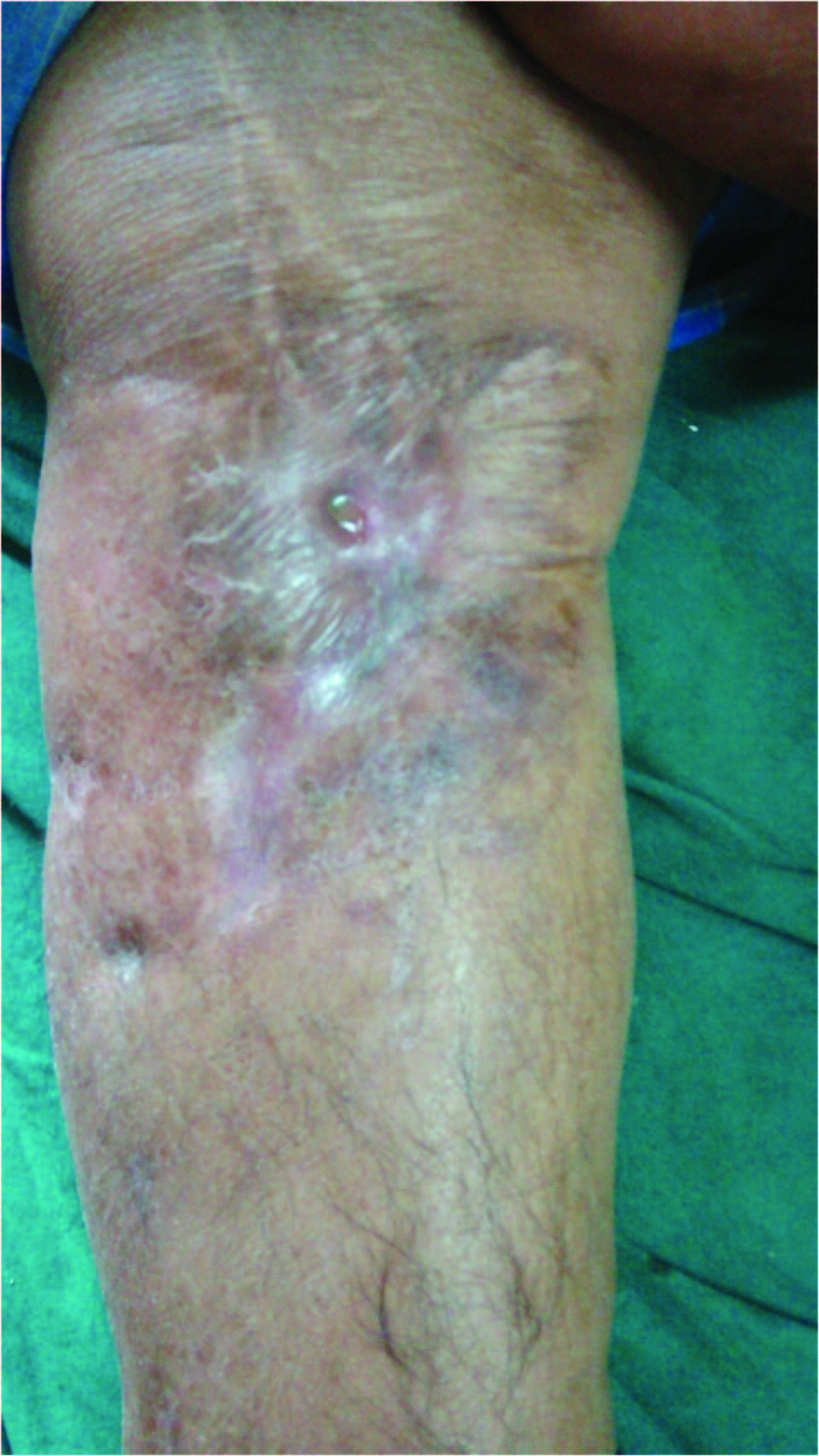
A 56-year-old non-diabetic and normotensive male patient presented in the Department of Microbiology after being referred from Orthopaedics outpatient department (OPD) with a history of low grade fever, limping gait, pain and foul smelling purulent discharge from a sinus below the right knee for last six months and with a provisional diagnosis of chronic osteomyelitis from bacteriological investigations [Table/Fig-1]. He gave history of trauma five years ago for which he was operated and an implant was placed in-situ at the right proximal tibia. Six months back he developed pain with purulent discharge from a sinus below right knee insidiously. He received empirical treatment with intravenous ceftriaxone 2g once daily and oral levofloxacin 750mg for six weeks. Then, re- operation was done to remove the implant around four months back. But discharge did not subside. No history of any other focus of infection such as urinary tract infection, skin infection, lung infection or suppurative gastrointestinal infection was there in the patient. No history of any other systemic illness, haematologic abnormality or endocrinopathy has been discernible. Local examination revealed erythema, elevated temperature, limitation or restriction in knee extension and flexion. Systemic examination revealed nothing remarkable. Blood investigations were as follows: Hb-13.2 gm%, total leucocyte count- 8300/cumm, ESR- 55mm in the first hour (Westergren method), fasting blood glucose(FBS)- 102 mg/dL, urea- 49 mg/dl, creatinine- 1.27 mg/dl, total bilirubin- 0.72 mg/dL, conjugated bilirubin – 0.41 mg/dL, SGPT- 37 IU/ml, SGOT- 34 IU/ml, ALP-9.7 KA units. Digital X-ray of the right knee showed diffuse cystic and lytic lesions consistent with periosteal reaction over proximal tibia.
Lesion of osteomyelitis below right knee.
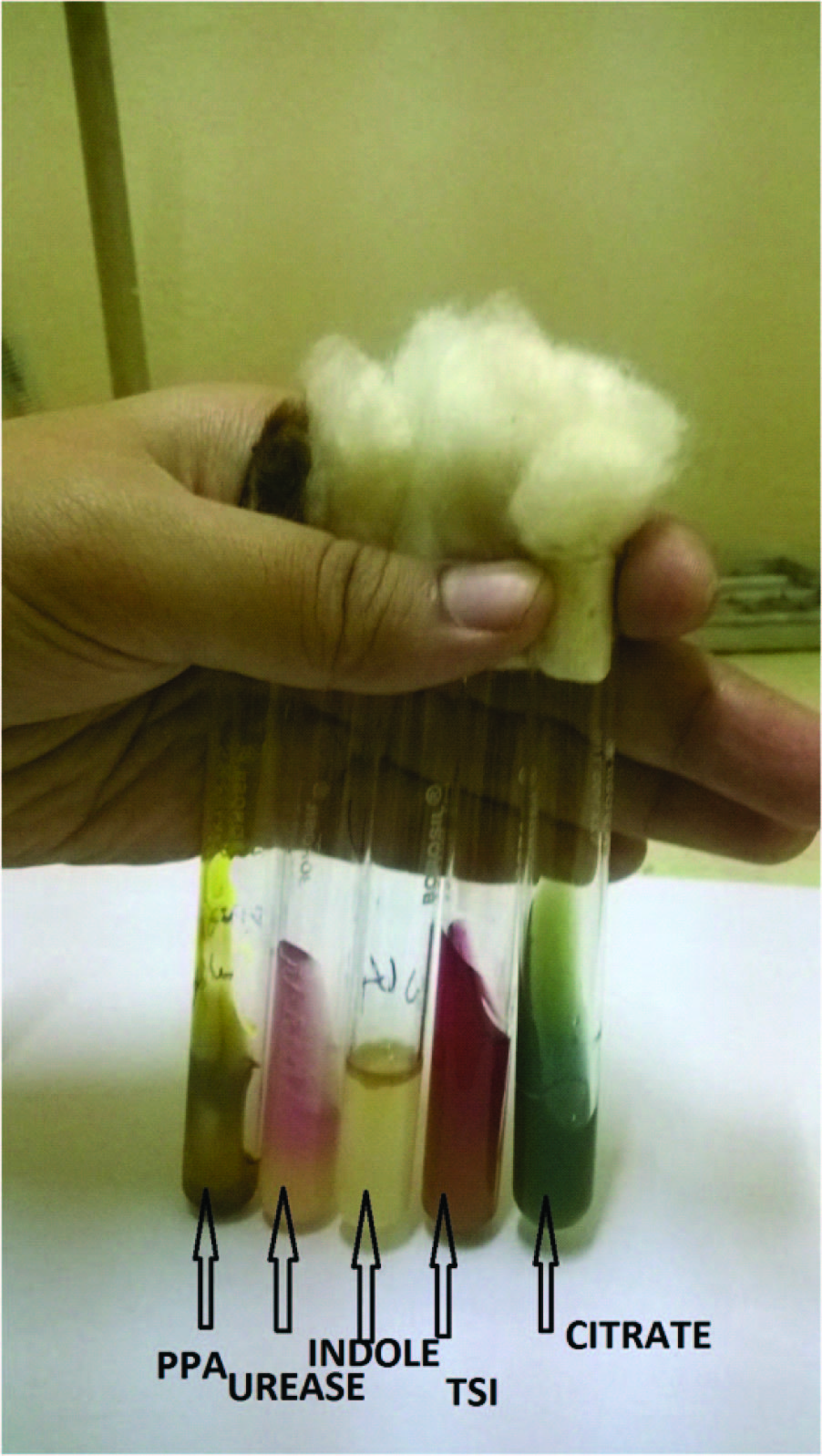
Two aliquots of pus were obtained aseptically from the sinus. One was subjected to microscopy after Gram staining and Z-N staining. Another was processed for bacteriological culture. It was incubated aerobically in 5% sheep blood agar and Mac Conkey agar at 370C for 24 hours. On microscopy, plenty of pus cells were found with few gram negative bacilli in Gram staining and no acid fast bacillus in Z-N staining. Culture showed pure growth of convex, pale non lactose fermenting colonies on Mac Conkey agar and there was no swarming on blood agar growth. Gram stain of colony revealed gram negative bacilli. Hanging drop test showed motile bacilli. Biochemical tests were performed on the isolated gram negative colonies. The organism was catalase and nitrate reductase positive, oxidase test negative. It was positive for tests of phenylalanine deaminase, urea hydrolysis, indole production, methyl red and ornithine decarboxylase. It was tested negative for production of gelatinase, arginine dihydrolase, lysine decarboxylase, beta-galactosidase and citrate utilization. However, it fermented mannose but none of the other carbohydrates commonly used for identification viz. d-mannose, adonitol, maltose, trehalose and xylose. Triple Sugar Iron (TSI) medium showed alkali/acid (K/A) reaction with no gas and H2S production [Table/Fig-2]. The organism was identified to be Morganella morganii. Antimicrobial sensitivity testing was done by modified Kirby Bauer disc diffusion method following CLSI 2014 document, Performance Standard for Antimicrobial Susceptibility Testing [1]. The isolate was found to be susceptible to amikacin, piperacillin-tazobactam, cefepime, levofloxacin, imipenem and resistant to ceftazidime, cefotaxime and polymixin B. The identity and AST pattern of the pathogen were further confirmed by vitek-2 system from an outside institution.
Biochemical tests for identification.
Blood culture was also put up for this patient with two sets of 10 ml of aseptically collected blood in each by conventional (manual) method. It showed no growth even after seven days of aerobic incubation.
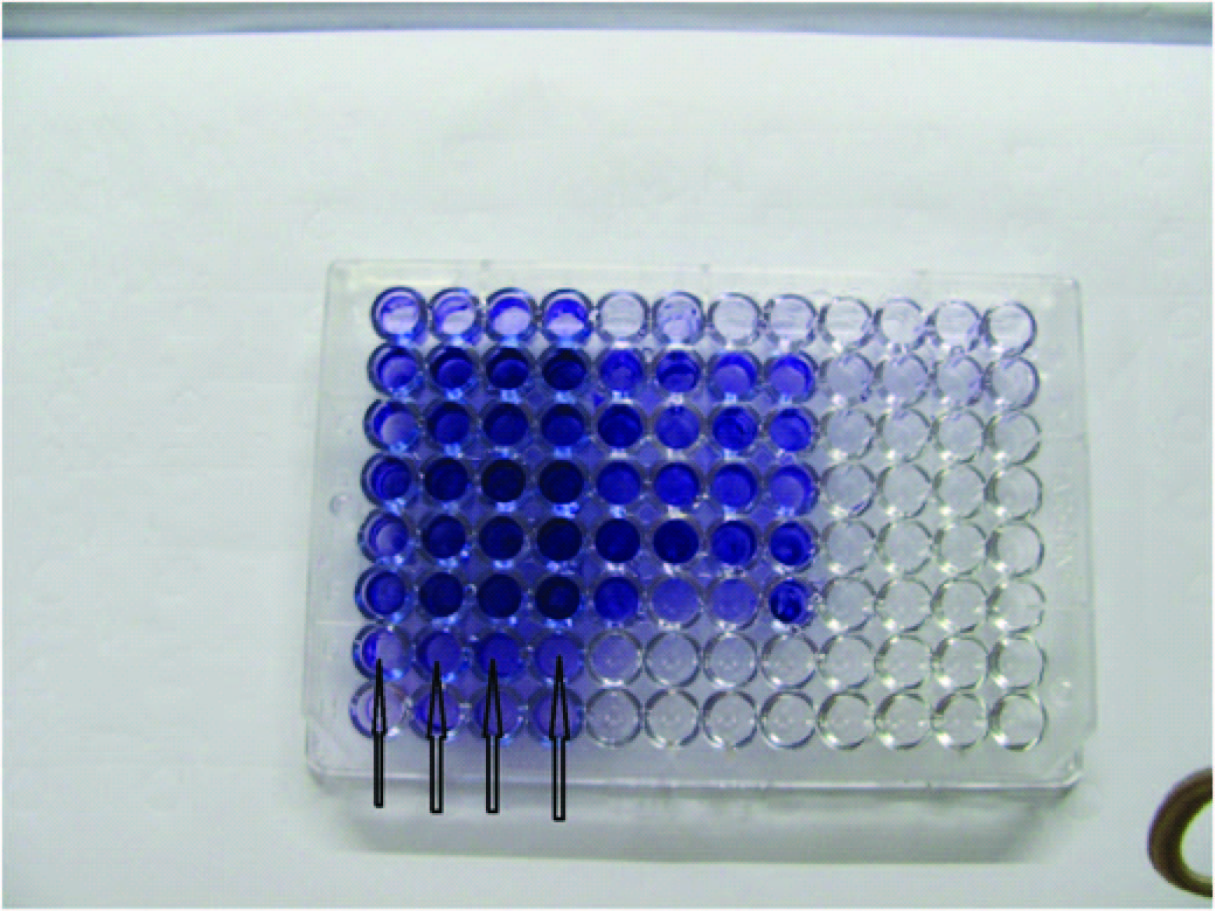
Biofilm production was detected by an in-vitro technique coined by Stepanovic et al., based on optical density (OD) determination [Table/Fig-3] [2]. The test organism showed average OD value of 0.256 where control OD value was 0.001. OD of test was >4 times than OD of control, therefore the test organism was a strong biofilm producer.
Microtitre plate method for detection of biofilm by Stepanovic et al., Row G (G1, G2, G3, G4) indicators of biofilm production by Morganella morganii.
Following culture antibiogram, the orthopedicians of the concerned patient were suggested to prescribe intravenous piperacillin –tazobactam combination and amikacin for six weeks. Moreover, from biofilm point of view they were reminded to consider surgical removal of affected bony and soft tissue segment for permanent cure followed by antimicrobial therapy for another four to six weeks. Consequently the patient underwent surgical removal of affected bone and soft tissue with bone and soft tissue grafting under thorough antimicrobial coverage before and after the procedures. Now, the patient is with no related problem out- standing, except a bad scar.
Discussion
Chronic osteomyelitis is the infection of the bone and bone marrow. Osteomyelitis cases can be classified by various criteria, including pathogenesis, duration of infection, location of infection, and presence or absence of foreign material. It can occur by contiguous spread or by haematogenous spread or by secondary infection in the setting of vascular insufficiency or concomitant neuropathy. Classification of osteomyelitis according to the duration of infection, although ill defined (because there is no clear time limit for the transition from acute to chronic osteomyelitis), is useful because the management of acute and chronic osteomyelitis differs. Chronic osteomyelitis lasts for weeks or months before treatment is started. Most common causative agent is Staphylococcus aureus in all age groups, followed by streptococci, gram-negative bacilli-mainly Escherichia coli and Pseudomonas aeruginosa. Less commonly encountered agents are Mycobacterium spp., Brucella spp. as well as anaerobic and fungal agents like Candida spp. Few other fungal agents as Histoplasma spp., Cryptococcus spp, Sporothrix schenckii, Blastomyces dermatitidis are rarely found in patients who live or have traveled in endemic regions [3]. Morganella morganii, a member of colonic microbiota causes opportunistic infections that particularly affects immunosuppressed patients, is not a common pathogen observed both in osteomyelitis and septic arthritis. Local events in the bony tissues e.g. trauma and suppressed systemic immune mechanism play an important role in the formation of bone infection [3]. Biofilm infections are typically insidious in onset, treatment refractory and can occur in immunocompetent individuals as well. Biofilm colonization is known to occur on dead bone, fibrosed tissues and sinus tracts in case of osteomyelitis [4]. Common commensal pathogens of skin such as Staphylococcus epidermidis are also strong biofilm producers.
Osteoarticular pathologies caused by Morganella morganii are rarely encountered. There have been very few cases reported in the literature, of which the majority includes sporadic septic arthritis without bony involvement in diabetic patients. Immunosuppression, long-term urinary catheterization, diabetes, rheumatoid arthritis, chronic alcoholism, prolonged corticosteroid therapy, malignancy, intravenous drug use, local trauma and surgical interventions are predisposing factors for Morganella morganii infection [5–11]. Patients with one or more of these risk factors demonstrate high mortality. Predisposing factors in our case were trauma followed by surgical implant. Biofilm colonization by the bacterial strain, probably invited more by surgical implant was an important pathogenic factor for chronicity of the infection. Impaired penetration of an antibiotic into the biofilm matrix, genetic diversity, quorum sensing, altered micro-environment within the biofilm (e.g.,: pH, O2 content) and reduced growth rate of bacteria in biofilms render the bacteria less susceptible to antibiotics in biofilm state and hence chronicity of the infection [12]. Therefore, inspite of the invitro AST the antimicrobials may not act accordingly due to the presence of biofilm mode of growth of the organism at site of infection and infections develop chronicity. Biofilm is thought to be responsible for many non-responding bacterial infections such as cystic fibrosis, pneumonia, musculo-skeletal infections, necrotizing fasciitis, osteomyelitis, meloidosis, infectious kidney stones, bacterial endocarditis, airway infections, otitis media, chronic rhinosinusitis, biliary tract infections, chronic bacterial prostatitis and infections related to medical devices [4]. We have found biofilm colonization by various organisms in some other infections e.g., non-healing port site infection, anal fistulae and chronic dacrocystitis. A study group from United States has reported biofilm colonization in osteomyelitis of jaw demonstrated by scanning electron microscopy and histopathology [13]. The main principles of osteomyelitis treatment are debridement of the necrotic tissues in a radical manner, filling up of the dead space and effective long-term antibiotic therapy which is ideal for biofilm infection. The antimicrobial treatment should not be empirical rather directed towards the isolated pathogen from the pus or tissue as in this case we recommended piperacillin-tazobactam and amikacin based on the AST report.
Conclusion
The case is rare and hence worth mentioning with regard to both the causative pathogen and biofilm production as well. Firstly, it can be inferred that Morganella morganii should be considered as a potential aetiological agent for chronic osteomyelitis. Secondly, biofilm producing strains of Morganella morganii may be considered pathogenic even in immuno-competent patients. Thirdly, isolation of uncommon pathogens from such chronic cases should prompt us to screen for biofilm producing capability of the concerned pathogens to cure the case radically instead of frequent investigations and change of antimicrobial therapy.
[1]. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Twenty-fourth Informational Supplement. Pennsylvania, Wayne, USA, 2014 [Google Scholar]
[2]. Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M, A modified microtiter-plate test for quantification of staphylococcal biofilm formationJ Microbiol Methods 2000 40(2):175-79. [Google Scholar]
[3]. Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J, Harrison’s principles of internal medicine 2015 19th edNew YorkMc Graw Hill Medical:838-45. [Google Scholar]
[4]. Costerton JW, Stewart PS, Greenberg EP, Bacterial biofilms: A common cause of persistent infectionsScience 1999 284(5418):1318-22. [Google Scholar]
[5]. Koyuncu W, Ozan F, Morganella morganii osteomyelitis complicated by secondary septic knee arthritis: a case reportActa Orthop Traumatol Turc 2012 46(6):464-67. [Google Scholar]
[6]. Katz LM, Lewis RJ, Borenstein DG, Successful joint arthroplasty following Proteus morganii (Morganella morganii) septic arthritis: a four-year studyArthritis Rheum 1987 30:583-85. [Google Scholar]
[7]. Cetin M, Ocak S, Kuvandik G, Aslan B, Temiz M, Aslan A, Morganella morganii-associated arthritis in a diabetic patientAdv Ther 2008 25:240-44. [Google Scholar]
[8]. Gautam V, Gupta V, Joshi RM, Sawhney G, Duhan S, Morganella morganii–associated arthritis in a diabetic patientJ Clin Microbiol 2003 41:3451 [Google Scholar]
[9]. Smithson Amat A, Perelló Carbonell R, Arenillas Rocha L, Soriano Viladomiu A, Osteomyelitis of the rib due to Morganella morganiiAn Med Interna 2004 21:464 [Google Scholar]
[10]. Schonweter RS, Orson FM, Chronic Morganella morganii arthritis in an elderly patientJ Clin Microbiol 1988 26:1414-15. [Google Scholar]
[11]. Deutsch M, Foutris A, Dourakis SP, Mantzoukis D, Alexopoulou A, Archimandritis AJ, Morganella morganii associated acute osteomyelitis in a patient with diabetes mellitusInfectious Diseases in Clinical Practice 2006 14:123 [Google Scholar]
[12]. Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G, The application of biofilm science to the study and control of chronic bacterial infectionsJ Clin Invest 2003 112(10):1466-77. [Google Scholar]
[13]. Sedghizadeh PP, Kumar SKS, Gorur A, Schaudinn C, Schuler CF, Costerton W, Microbial biofilm and osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapyJ Am Dent Assoc 2009 140:1259-65. [Google Scholar]