Postoperative pain management is a necessary component of patient undergoing major surgery. Adequate pain management is a challenge to the pain physician as there are many adverse effects of psychological and physiological effects associated with pain [1]. Pain can hamper the normal recovery process and extend the length of hospital stay, contribute towards patient dissatisfaction, cause a negative perception of hospital performance and increase the health care utilization costs. Multimodal approach and drugs have been used for pain control with variable achievement. Every technique and drug has its own advantages and disadvantages. Buprenorphine is a semi synthetic derivative of thebaine, a morphine alkaloid, being a potent and safe analgesic (75 to 100 times greater than that of morphine), at 5-10% receptors occupancy, causing less respiratory depression [2–5]. The Transdermal Delivery Systems (TDS) has been use in clinical practice, they overcome the pharmacokinetic problems of oral and parenteral routes [6].
Buprenorphine is homogeneously incorporated in solid polymer matrix patch which is applied to skin. The adhesive buprenorphine patch is non-invasive slowly and continuously releases the drug into the systemic circulation. Transdermal drug delivery systems are simple and compliant methods of delivery [7,8]. They are designed to provide sustained drug release for prolonged period. These characteristics suggest that transdermal buprenorphine may be useful in the postoperative pain management of patient undergoing lower abdomen surgeries. Tramadol is centrally acting atypical opioid analgesic with additional serotonin nor-epinephrine reuptake-inhibiting effects [9]. It is marketed as a racemic mixture of both R and S stereoisomers and the two isomers complement each other’s analgesic activity [9,10].
In this study, we aimed to evaluate the efficacy and haemodynamic effects of transdermal buprenorphine patch (10mg and 20mg) in postoperative pain management for lower abdominal surgery.
Materials and Methods
A prospective randomized, double-blind controlled study was performed after obtaining the approval of the Ethical Committee of the institution. This study was conducted (July 2013 to August 2014) in patients for elective abdominal surgery under general anaesthesia between the age group of 18-55 years of either sex within ASA Grade I-II. Patients with skin allergy, ASA grade III and IV, refusal to consent and pregnant patients were excluded. Informed written consent was obtained from all subjects. Patients were randomized into three groups (n=30 in each group) using a computer generated random number tables. These groups were further classified, based on the TDS drug combination (patches) used with injection tramadol (1-2 mg/kg body weight).
Group A: Placebo patch (32x32 mm Sparsha Pharma).
Group B: Transdermal patch of buprenorphine 10mg.
Group C: Transdermal patch of buprenorphine 20mg.
All patients were premedicated on the night before surgery with ranitidine 150 mg, metaclopromide 10mg and Alprazolam 0.25 mg orally. Transdermal patch was applied to hair-less sites, with most common sites being the upper outer arm, chest, upper back, or side of the chest night before surgery. Intraoperative monitoring included non-invasive arterial blood pressure, electrocardiography, capnography, pulse oximetry and temperature. All the base line parameters (heart rate, Blood pressure, SpO2, ETCO2) were recorded and intravenous line was obtained with 18 gouge cannula on the dorsum of hand. All the patients were premedicated with inj. glycopyrrolate (0.2mg), inj.ondansetran (4mg), inj.midazolam (0.07 - 0.15mg/kg) and inj. fentanyl (2μgm/kg) i.v. After pre oxygenation, the patients were induced intravenously with inj. propofol (2mg/kg) and succinylcholine (1.2mg/kg) to facilitate tracheal intubation. Anaesthesia was maintained with 60% N2O and 40% O2 with isoflurane, adequate muscle relaxant (vecuronium bromide) and ventilation was adjusted to maintain end tidal CO2between 35 to 40 mmHg. At the end of the surgery, the residual muscles relaxation was reversed, using an inj. neostigmine (0.04mg/kg – 0.08mg/kg) and glycopyrrolate (0.005 – 0.01mg/kg). After extubation VAS score was assessed and if score was more than 2, Inj. tramadol i.v. was given as a rescue analgesic. VAS scores were assessed at 2hr, 6hr, 12hr, 2nd day - 7th days after surgery and Inj. tramadol was given whenever VAS score was found to be ≥2. Ramsay score was used for determination sedation of patients. Sedation was assessed after extubation. Complications such as nausea, vomiting, application site rashes and pruritus, headache and constipation were noted and treated accordingly. An anaesthesiologist who was blinded to the study drugs documented all the parameters.
Statistical Analysis
Continuous data were summarized as mean ± SD while discrete (categorical) data in percentage (%). Continuous covariates were compared using analysis of variance (ANOVA) followed by Tukey’s post-hoc test. The categorical variables were compared by chi-square test. p<0.05 was considered statistically significant.
Results
There were no significant differences between groups in demographic data (age, gender), and ASA-I/II (p-value >0.05) [Table/Fig-1].
Demographic profile of study population.
| Group A (n=30) | Group B(n=30) | Group CB(n=30) | p- value |
|---|
| Age (Mean±SD) | 35.87±9.71 | 35.80±10.11 | 39.3±10.37 | 0.27 |
| GenderFemaleMale | 15 (50.0%)15 (50.0%) | 21 (70.0%)9 (30.0%) | 23 (76.7%)7 (23.3%) | 0.077 |
| ASA I/II | 18/12 | 17/13 | 19/11 | >0.05 |
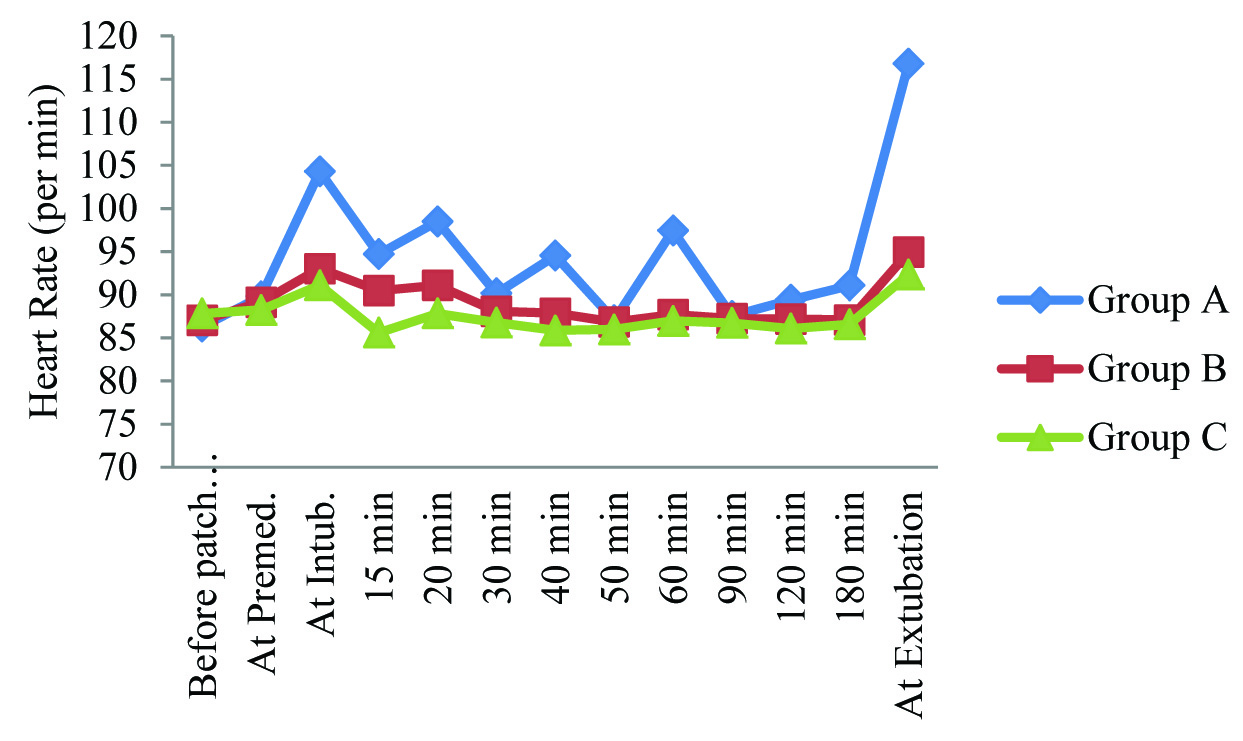
Heart rate of Group A was found to be higher than other groups, at all-time intervals except at baseline (before patch application). Heart rate of Group B was found to be higher than that of Group C at all-time intervals except at baseline (before patch). This intergroup difference in heart rate was found to be statistically significant only at the time of intubation, at 15 min, 20 min, 40 min, and 60 min after intubation and at the time of extubation [Table/Fig-2].
Summary of heart rate (per min) in study population
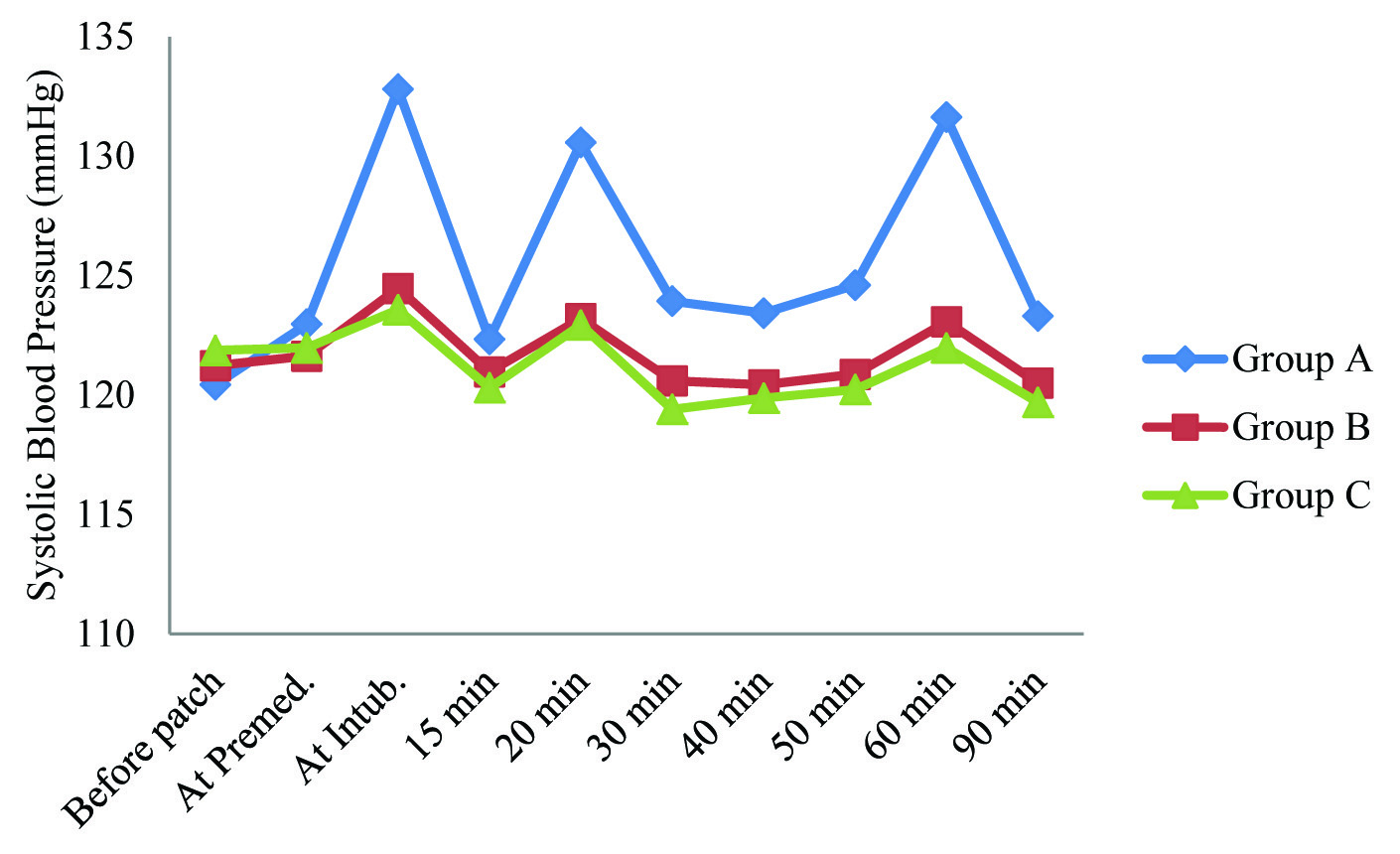
Systolic blood pressure of Group A was found to be higher than other groups, at all-time intervals except at baseline (before patch application). Systolic blood pressure of Group B was found to be higher than that of Group C at all-time intervals except at baseline (before patch) and at premedication. This intergroup difference in Systolic blood pressure was found to be statistically significant only at the time of intubation, at 20 min, 60 min after intubation and at the time of extubation [Table/Fig-3].
Summary of Systolic blood pressure (mm hg) in study population
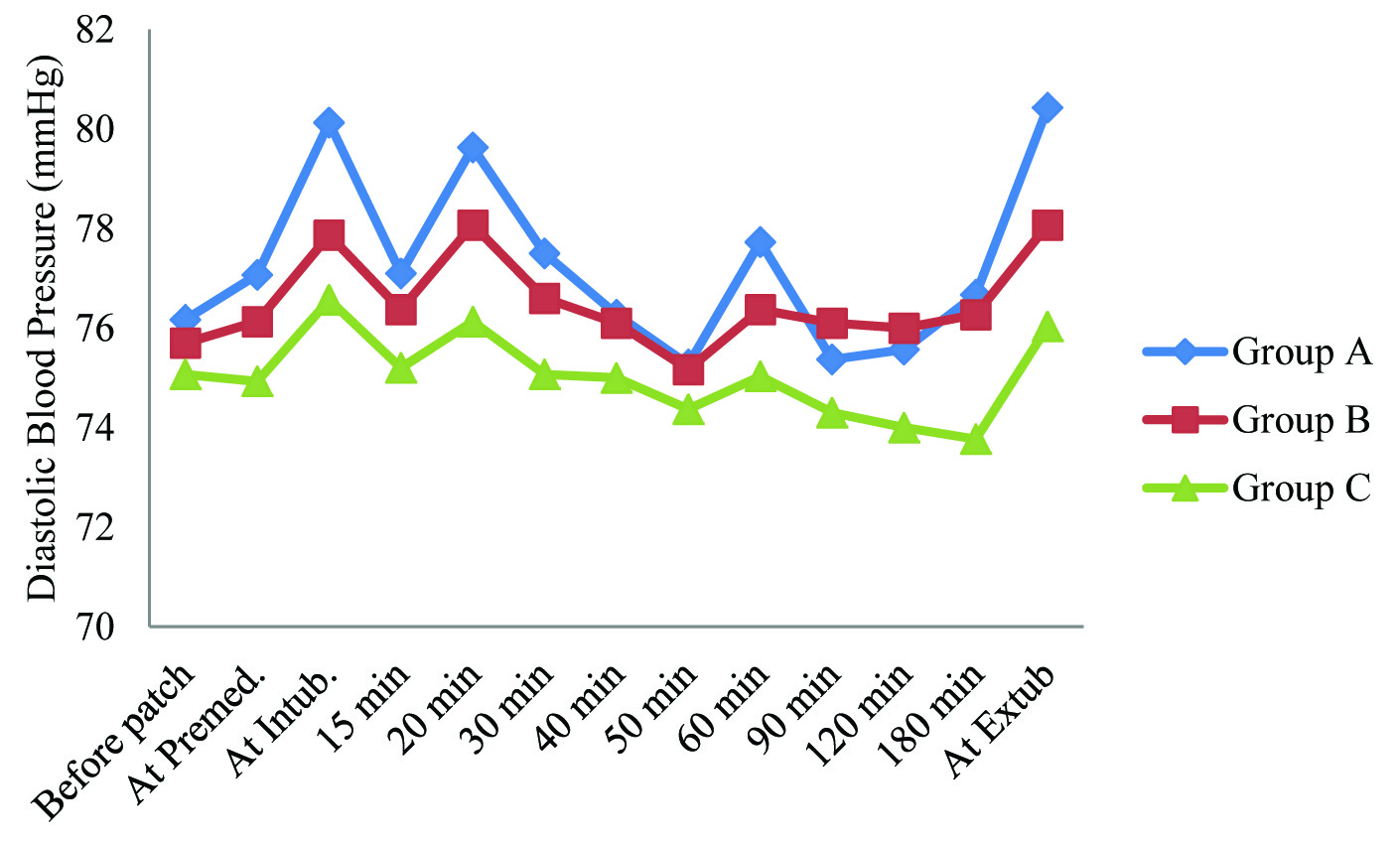
Diastolic blood pressure of Group A was found to be higher than other groups, at all-time intervals except at 90 min and 120 min after intubation. Diastolic blood pressure of Group B was found to be higher than that of Group C at all-time intervals. This intergroup difference in diastolic blood pressure was found to be statistically significant only at the time of extubation [Table/Fig-4].
Summary of diastolic blood pressure (mm hg) in study population
At the end of surgery VAS scores as reported by Group A subjects were found to be of higher order (4.93±0.98) as compared to Group B (1.73±0.64) and Group C (1.40±0.50). At all-time durations, VAS scores of Group A were found to be higher than other groups. VAS scores of Group B were found to be of higher order than that of Group C. Difference in VAS scores among the three groups found to be statistically significant at all-time intervals. On 2nd day after surgery, no pain was reported by the Group C patients and on 4th day after surgery, no pain was reported by Group B patients [Table/Fig-5].
Intergroup comparison of vas score in study population.
| Time intervals | Group A(Mean±SD) | Group B(Mean±SD) | Group C(Mean±SD) | p-value |
|---|
| At end of surgery | 4.93±0.98 | 1.73±0.64 | 1.40±0.50 | <0.001 |
| 2 hour | 4.27±0.74 | 1.23±0.50 | 0.77±0.43 | <0.001 |
| 6 hours | 5.03±0.96 | 1.03±0.18 | 0.63±0.49 | <0.001 |
| 12 hours | 3.67±0.84 | 0.83±0.38 | 0.67±0.48 | <0.001 |
| 2nd day | 3.43±0.63 | 0.33±0.48 | 0.00±0.00 | <0.001 |
| 3rd day | 2.83±0.53 | 0.03±0.18 | 0.00±0.00 | <0.001 |
| 4th day | 2.40±0.56 | 0.00±0.00 | 0.00±0.00 | <0.001 |
| 5th day | 1.43±0.50 | 0.00±0.00 | 0.00±0.00 | <0.001 |
| 6th day | 1.63±0.61 | 0.00±0.00 | 0.00±0.00 | <0.001 |
| 7th day | 0.70±0.60 | 0.00±0.00 | 0.00±0.00 | <0.001 |
At the end of surgery, mean RSS score in Group A (1.13±0.35) was found to be lowest followed by that of Group B (2.13±0.43) and highest for Group C (2.70±0.47) and this difference was found to be statistically significant (p<0.001). Similar trend of RSS was found at 2, 6 and 12 hours after the surgery. At 2 days after surgery, RSS scores of Group A and Group B were found to be (2.00±0.00) i.e. all the patients of Group A and Group B had RSS score of 2 which was lower than that in Group C (2.03±0.18) but this difference was not found to be statistically significant (p=0.368). At 3rd day onwards up till day 7, RSS score of 2 was observed in all patients of Group A, Group B and Group C and no difference in RSS score was observed during this period [Table/Fig-6].
Intergroup comparison of RSS score in study population.
| Time intervals | Group A(Mean±SD) | Group B(Mean±SD) | Group C(Mean±SD) | p-Value |
|---|
| At end of surgery | 1.13±0.35 | 2.13±0.43 | 2.70±0.47 | <0.001 |
| 2 hour | 1.17±0.38 | 2.00±0.00 | 2.53±0.51 | <0.001 |
| 6 hours | 1.30±0.47 | 2.00±0.00 | 2.33±0.48 | <0.001 |
| 12 hours | 1.73±0.45 | 2.00±0.00 | 2.07±0.25 | <0.001 |
| 2 days | 2.00±0.00 | 2.00±0.00 | 2.03±0.18 | 0.368 |
| 3 days | 2.00±0.00 | 2.00±0.00 | 2.00±0.00 | 1.000 |
| 4 day | 2.00±0.00 | 2.00±0.00 | 2.00±0.00 | 1.000 |
| 5 day | 2.00±0.00 | 2.00±0.00 | 2.00±0.00 | 1.000 |
| 6 day | 2.00±0.00 | 2.00±0.00 | 2.00±0.00 | 1.000 |
| 7 day | 2.00±0.00 | 2.00±0.00 | 2.00±0.00 | 1.000 |
No postoperative complications were found in higher proportion in Group C (90.00%) and Group B (86.67%) as compared to Group A (80.00%). Nausea was the most common complication and its prevalence was higher in Group A (16.67%) as compared to Group B (10.00%) and Group C (10.00%). Difference in complications found in above three groups was not found to be statistically significant (p>0.05) [Table/Fig-7].
Post-operative complications.
| Complications | Group A | Group B | Group C | p-value |
|---|
| No. | % | No. | % | No. | % |
|---|
| Nausea | 5 | 16.67 | 3 | 10.00 | 3 | 10.00 | 0.67 |
| Vomiting | 1 | 3.33 | 1 | 3.33 | 0 | 0.00 | 0.61 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Application site rashes | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Headache | 0 | 0 | 0 | 0 | 0 | 0 | - |
| No complication | 24 | 80.00 | 26 | 86.67 | 27 | 90.00 | 0.54 |
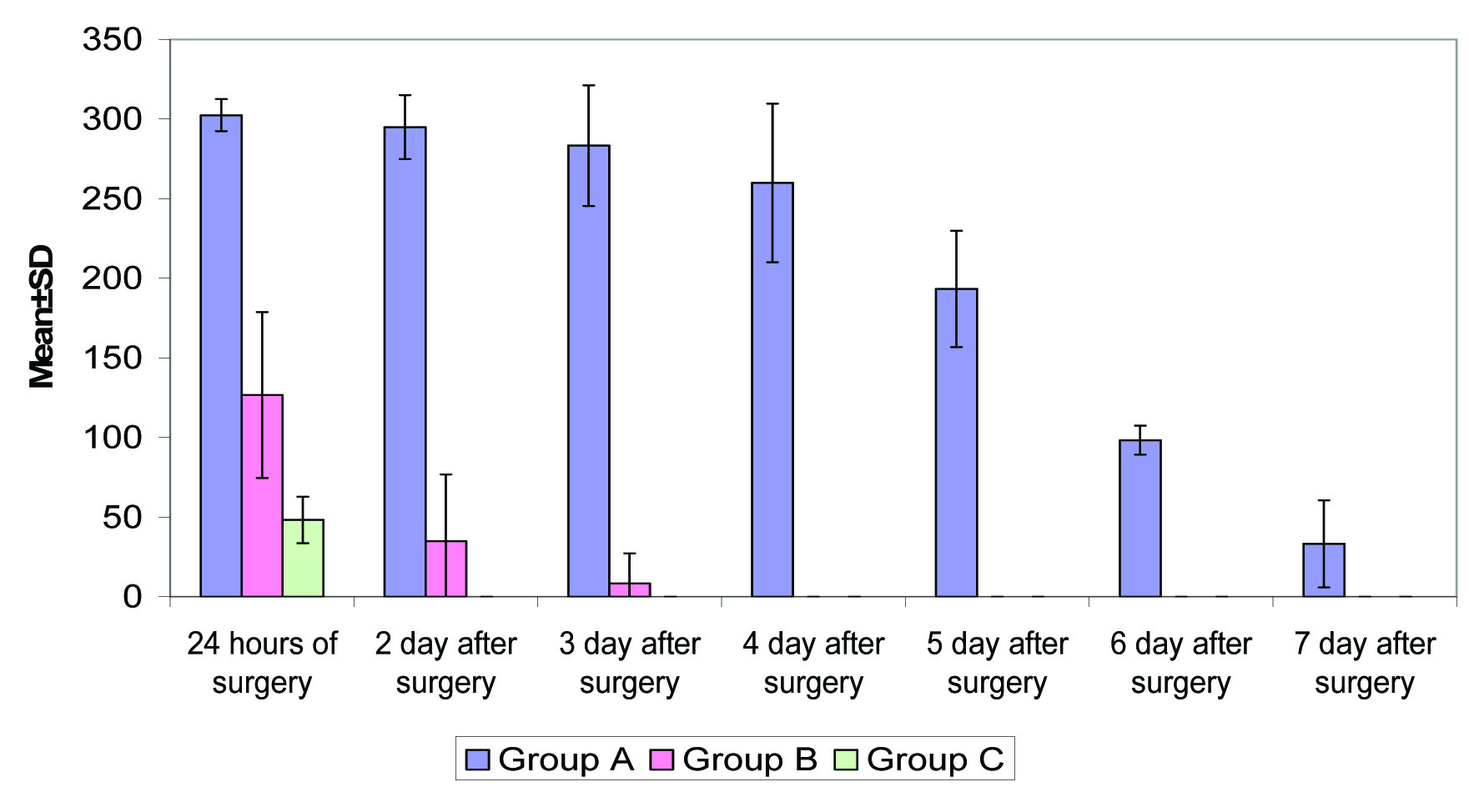
Within first 24 hours of surgery analgesia requirement in Group A was highest (302.50±10.06 mg) followed by that in Group B (126.67±52.08 mg) and least in Group C (48.33±14.58 mg) and this difference was found to be statistically significant (p<0.001). On 2nd day of surgery, the analgesia requirement in Group A was highest (295.00±20.13 mg) followed by in Group B (35.00±41.83 mg) whereas in Group C no analgesia was required. On 4th postoperative day, no analgesia was required in Group B subjects as well. Difference in analgesia requirement of above three groups was found to be statistically significant at all above time intervals as shown in [Table/Fig-8].
Postoperative Analgesia Requirement (amount in mg).
Discussion
Post-surgical pain is a complex response to trauma during surgery that stimulates the central nervous system. It raises the possibility of complications, cost of medical care and healing recovery [11]. Transdermal Drug Delivery System (TDDS) provides safe, convenient and reliable method of drug delivery. It is a preferable alternative to oral and parenteral drug delivery method as it avoids painful skin punctures and multiple dosing. TDS has an advantage in having a high tendency for first-pass metabolism. These can be release in small doses with a sustained blood level [12].
In the present study the heart rate and Systolic Blood Pressure (SBP) of group A, receiving placebo patch, were found to be significantly higher than group B and C at most time intervals except at baseline. We also found that the peak-effect of buprenorphine patch is achieved at 12-24 hours. Efficacy of buprenorphine transdermal patch was dosage dependent; mainly in the first postoperative hour where additional analgesia was required [13]. The peak-effect of buprenorphine patch was achieved at 12-24 hours, so the patients remained heamodynamicaly stable at intubation and thereafter [14]. Oifa et al., also reported that the buprenorphine infusion (BUP-i) and buprenorphine bolus (BUP-b) in abdominal surgery patients provide haemodynamically stable during intra and postoperative period [15]. The systolic blood pressure was recorded; if baseline blood pressure more than 20% fall than one bolus dose of ephedrine (6 mg) was given. Setti et al., also found that buprenorphine transdermal delivery system (BUP-TDS) efficacy was directly proportional to its dosage [13]. No significant differences in Diastolic Blood Pressure (DBP) were observed amongst all the three groups except at the time of extubation. Although statistically significant, changes were physiologically not relevant and of no clinical importance.
In our study, the group A patients were found to have higher VAS Score at end of surgery than group B and group C. Differences in VAS Score among the three groups at the end of surgery was found to be statistically significant at all intervals. It was found that VAS score became progressively lesser in group B and group C as compared to group A postoperatively and the intermittent rescue analgesic requirement was more in group A as compared to group B and C. VAS score and postoperative rescue analgesic requirement was significantly lower in group C. The findings of our study are supported by Arshad et al., who reported that the buprenorphine TDS was significantly decreasing postsurgical pain [8].
In the present study the rescue analgesia was required in all groups. The difference in analgesia requirement of above three groups was found to be statistically significant at all above time intervals i.e. 24 hours postoperatively and thereafter, second, third, fourth, fifth, sixth and seventh day after surgery. The findings of our study are supported by Setti et al., Böhme and Likar who reported that the efficacy of transdermal buprenorphine patches was directly proportional to its dosage, although additional analgesia was required, particularly in the 1st postoperative hour in gynecological surgery [13,16]. In a previous study Sittl et al., had found that patients treated with transdermal buprenorphine had reduced need for additional analgesics [4].
In our study, we found that nausea was the most common side effects and its prevalence was higher in group A as compared to group B and group C and thus was not statistically significant. Other treatment related side effects such as, headache, application site pruritus, application site erythema, application site rash, dizziness, constipation and dry mouth, were not found in our study. Walsh et al., observed that the nausea, vomiting, euphoria, sedation, delayed gastric emptying and pupillary constriction were all seen to a lesser degree with buprenorphine as compared to morphine [17]. This was probably due to the high lipophilicity of buprenorphine as compared to morphine. However, Gordon et al., observed that patients had more adverse events when they received buprenorphine transdermal patches than when they received placebo [18]. Buprenorphine transdermal patch was significantly associated with nausea, vomiting, somnolence, dry mouth and dizziness whereas constipation was not significantly associated with buprenorphine transdermal patch as compared with placebo. The possible reason could be that, in our study tramadol was used as a rescue analgesic and the requirement of tramadol was more in group A which itself causes nausea.
Conclusion
Transdermal Buprenorphine patch (20 mg) is effective in attenuating postoperative pain, maintaining haemodynamic stability and fewer postoperative rescue analgesic requirements, for patients undergoing lower abdominal surgeries under general anaesthesia. Further studies with greater sample size will be essential to approve this.