Few studies have mentioned the advantage of Real-Time Polymerase Chain Reaction (PCR) in bringing important contribution to the detection of pathogenic bacteria in mixed oral infections [11–14]. Hence, in the present study detection of P. gingivalis fimA type I genotype was performed by real-time PCR assay.
Materials and Methods
Study population: This comparative study comprised 23 chronic marginal gingivitis patients. Chronic generalized periodontitis (n=23) were included as control group. The subgingival plaque samples from the two groups were collected from the Department of Periodontics and Implantology, Sree Balaji Dental College and Hospital, Chennai. The period of study was from July 2014 to January 2015. The study was approved by the institutional ethics committee, Dr A.L.M Post Graduate Institute of Basic Medical Sciences, University of Madras, Chennai. Informed consent was obtained from both the study subjects.
Subject Selection (Inclusion & Exclusion Criteria): Either sex, presence of no less than 14 teeth, a minimum three teeth with probing depth >4 mm, Bleeding On Probing (BOP) were the inclusion criteria for chronic periodontitis patients. The chronic marginal gingivitis group was selected by measuring Gingival Index Score [15]. Gingivitis patients showing symptoms of chronic generalized marginal gingivitis with probing depth 3mm and Gingival Index Score as classified by LÖe and Silness for mild to moderate condition were recruited for the study population. The exclusion criteria for both the groups included pregnancy, lactation, antibiotic and antiinflammatory therapy for the past six months, smoking, paan chewing, presence of diabetes or other systemic illness and periodontal therapy for the past one year.
Sample Collection: The Probing Depth (PD) and Clinical Attachment Level (CAL) (mm) was measured with a graduated William’s probe. SubGingival Plaque (SGP) samples were collected from the mesio-buccal aspect of the tooth from three different sites and pooled in 500 μl of freshly prepared Phosphate Buffered Saline (PBS). Following the removal of supragingival plaque with the help of sterile cotton roll, the SGP was collected using Gracey curette from chronic periodontitis patients and chronic gingivitis subjects [16]. The samples were then transported in ice to the laboratory and stored at -20oC until assayed.
DNA Extraction
The subgingival plaque samples in PBS were transferred to fresh Eppendorf tube containing 100μl of lysis buffer(10mmol/L Tris-HCl, 1.0mmol/L EDTA, 1.0% Triton X-100, pH 8.0) following centrifugation and boiled for 10 minutes. The samples were then centrifuged and 10μl of the supernatant was used as template for PCR assay [17]. The extracted samples were stored at -20oC until assayed.
PCR Primer Designing: Primers targeting the fimA type I gene of P. gingivalis spanning positions 190455-190600 (forward 5’-CGAATCAAAGGTGGCTAAGTTGACCG-3’,reverse5’ GAGTCTTGCCAACCAGTTCCATTGC -3’) were designed based on the complete gene sequence acquired from the Gen Bank database (NCBI) (accession no: GI 3005672). The length of the amplicon is 170 bp. Sequences were aligned with ClustalW2 (Multiple sequence alignment) and searched for conserved domains (http://www.ebi.ac.uk). The oligocalculator tool (http://trishul.sci. gu.edu.au/tools/OligoCalculator.html) was used for checking the design of the candidate oligonucleotide sequences for tm, Guanine-Cytosine (GC) content and primer dimers. Validation of primer specificity was performed by submitting the primer sequences to the BLASTN program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and it was found that the primers possessed100% nucleotide identity and coverage threshold with the corresponding taxa. In silico PCR was performed using the NCBI primer designing tool (hhttp://www. ncbi.nlm.nih.gov/tools/primer-blast/) and it was observed that the primers were specific only for the intended target.
Real-time PCR assay for detection of P. gingivalis fimA Type I gene
Optimization of the Real-time PCR Assay: Reactions were conducted in an Applied Biosystems 7900 HT Fast Real-time PCR system, USA. SDS 2.3 software was used for conducting the experiment. Reaction optimization was performed with primer concentrations as stated by the kit manufacturer (Quantifast SYBR Green PCR Kit, QIAGEN GmbH, Hilden). Optimal reactions were performed in total volumes of 20μl containing 4μl DNA, 10μl of 2x QuantiFast SYBR Green PCR Master Mix and 0.5μM of each primer. PCR was carried out using the following conditions: Stage 1- 50oC for 2 minutes, Stage 2- 95oC for 10 minutes, Stage 3- 40 cycles of 95oC for 0.15 seconds, 60oC for 1 minute, Stage 4- 95oC for 0.15 seconds, 60oC for 0.15 seconds and 95oC for 0.15 seconds.
Data was collected at all stages. No Template Control (NTC) was included that contained all the components of the reaction except the template to rule out any contamination. Real-time PCR amplification assays were conducted in duplicate.
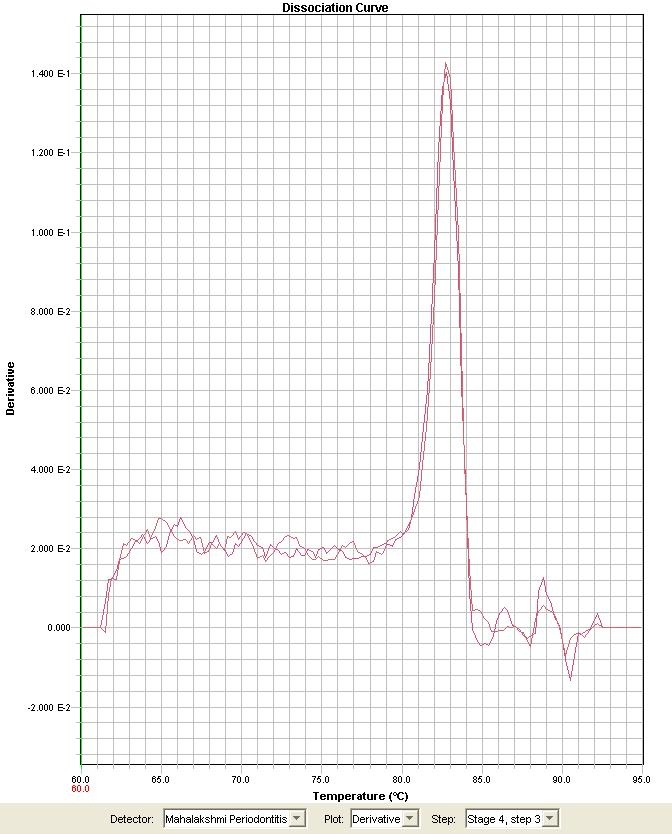
Melting Curve Analysis: Reaction specificities were verified by melting curve analysis with a progressive temperature increase from 60oC to 95oC at a 0.1oC/s transition rate and continuous fluorescence acquisition. To minimize potential primer-dimer artifacts during the analysis of clinical samples, fluorescence acquisition temperatures were set to approximately 4oC below the denaturing temperature of each amplification product, as previously established by melting curve analysis [Table/Fig-1]. Only 10μl of each real-time PCR product was subjected to 2.0% agarose gel electrophoresis, stained with ethidium bromide, and visualized under ultraviolet light to verify the size (170 bp) of the amplicon.
Melting curve analysis for P. gingivalis fimA type I gene with SYBR green dye.
Fig shows the well-defined unique peaks in the dissociation profiles with the absence of primer dimer formation.
Sequencing of Selected PCR Product: A representative amplified product of P. gingivalis type I fimA gene was sequenced by genome biotech, Pune [Applied Biosystem (ABI) 3130 Genetic Analyser, ABI PRISM Big Dye Terminators V 3.1]. Nucleotide sequences were visualized with Bioedit and submitted to the BLASTN program for comparison with sequences from the GenBank database (http://www.ncbi.nlm.nih.gov/genbank). A 99% minimum nucleotide identity was required for positive identification at the species level. The sequences of P. gingivalis fimA type I was submitted to GenBank under accession no. HQ706664.
Statistical Analysis
Standard deviation for all the samples was obtained by the SDS 2.3 software in the Applied Biosystems 7900 HT Fast Real-time PCR system, USA. The samples showing standard deviation value of ≤ 0.1 was recorded positive. The odds ratio was calculated using chi-square test with 95% confidence interval to analyze the association of P. gingivalis fimA type I genotype to chronic periodontitis and chronic gingivitis. The p-value <0.05 was considered statistically significant. Fisher’s exact test was used to calculate the p value for the prevalence of P. gingivalis fimA type I in the two groups. Unpaired t-test was used to compare the means of age, PD and CAL.
Results
[Table/Fig-2] depicts the results of real - time PCR assay and the clinical parameters of the gingivitis and chronic periodontitis group. Statistical analysis revealed insignificance (p=0.4) between the two groups with respect to male: female ratio. The subjects with periodontitis presented significantly higher mean PD (p<0.0001) and CAL (p<0.0001), than subjects with marginal gingivitis.
Clinical parameters/results of Real-time PCR of chronic periodontitis and chronic marginal gingivitis subjects.
| Chronic Periodontitis | Marginal gingivitis | p value |
|---|
| Age ¥ | 37.13±12.03 | 35.30 ± 10.13 | 0.0001** |
| Gender%(Male/Female)§ | 19/4 | 16/7 | 0.4 |
| Mean PD(mm) ¥ | 5.27±1.00 | 2.17±0.38 | 0.0001** |
| Mean CAL(mm) ¥ | 6.28±1.32 | 3.21±0.42 | 0.0001** |
| Real-time PCR (no of positive samples)& | 2 | 7 | 0.06& |
| Odds Ratio | 0.21 | 4.59 | |
§ Refers to Chi-square test; ¥ Unpaired t-test; & Fisher’s exact test; ** significant p value.
Out of the 23 SGP samples of chronic periodontitis patients, two showed amplification of specific P. gingivalis fimA type I gene after 30 cycles (Standard deviation for two samples = 0.24724083 & 0.49709812). Among 23 SGP samples of chronic gingivitis subjects, seven samples were positive for P. gingivalis fimA type I genotype and the number of cycles required to amplify P. gingivalis fimA type I gene in them ranged between 24 - 30 cycles (Standard deviation for seven samples = 0.23106992, 0.8157472, 0.083577536, 0.20680279, 0.64406186, 0.8770159, 0.62718827 & 0.159102). Among the seven positive samples of chronic gingivitis group two samples amplified following 26 cycles, a further two samples after 25 cycles, one sample each after 24, 28 and 31 cycles respectively. Evidence of nonspecific or cross-reaction products was not observed in the assay. The association between two study groups with respect to the outcome for P. gingivalis fimA type I genotype was considered to be statistically insignificant (p=0.06). Nevertheless the odds ratio tested showed an association of P. gingivalis fimA type I genotype to chronic marginal gingivitis. Statistical significance was observed between two groups in regard to pocket probing depth and clinical attachment level. The mean pocket probing depth and clinical attachment level for the P. gingivalis fimA type I gene positive samples among chronic periodontitis was 4.95 ± 0.91 and 5.7500 ± 1.76 respectively. While, the mean pocket probing depth and clinical attachment level in gingivitis was 2.10±0.27 and 3.01±0.23 respectively.
Discussion
Real-time PCR is a DNA amplification technique that allows precise determination of nucleic acid levels by monitoring fluorescent signals at a cycle-to-cycle rate [18]. As SYBR Green I can bind even to non specific double-stranded DNA, the reaction was standardized to evade such non specific product detection. In this regard, we have used preventive procedures to minimize such technical limitation, including preliminary evaluation of primer sequences and melting curve analysis of amplification products. The evidence of well-defined unique peaks in the dissociation profiles ensured satisfactory reaction specificities for all markers, with no evidence of primer-dimer formation. As an additional precaution, monitoring of reaction products was conducted by fluorescence acquisition at temperatures in which double-stranded DNA was presumably composed only by the target gene products. In the absence of the probe, the specificity of the SYBR Green Real-Time PCR was determined by the definition of the melting temperature of the PCR product obtained [Table/Fig-1]. Although a real-time PCR kit for the detection of P.gingivalisfimA type I gene is commercially available, in the present study we have designed a primer to detect the target gene. The gene sequence result reveals the primer pair target specificity.
The number of cycles required for the detection of P. gingivalis fimA type I genotype in marginal gingivitis was below 30 suggesting the presence of P. gingivalis fimA type I genotype in higher numbers among subjects with marginal gingivitis. While, among periodontitis this genotype was detected above 30 cycles. As there are hardly any reports available with regard to prevalence of P. gingivalis fimA type I genotype among adult gingivitis subjects, its comparison with diverse population was hampered. The high prevalence of fimA type I among adult gingivitis subjects is different from Hayashi et al., study who has reported a very low prevalence among children with gingivitis [19]. The low prevalence of P. gingivalis fimA type I genotype among chronic periodontitis was well in concurrence with observations of Van der Ploeg et al., Amano et al., and Fabrizi et al., [4,8,20]. Compared to the present study Beikler et al., [21], reported a high prevalence of P. gingivalis fimA type I genotype among Caucasian periodontitis patients. Amano et al., has reported a high prevalence of P. gingivalis fimA type I genotype in healthy subjects [4]. Alternatively two studies have reported type 1b as the second most prevalent genotype among periodontitis patients [9, 22]. Moreno et al., has reported P. gingivalis fimA type I genotype as the second most prevalent among health next to type II [23]. Conversely Hayashi et al., has reported a higher prevalence of Type II fimA among children with gingivitis [19].
Limitation
The main limitation of the study is lack of healthy group. Nevertheless several studies have reported the presence of P. gingivalis fimA type I genotype among healthy individuals.
Conclusion
In conclusion, the low prevalence of P. gingivalis fimA type I genotype among chronic periodontitis patients signifies its avirulence. The chronic marginal gingivitis patients who harbor this avirulent strain may not progress to periodontitis. Screening of this genotype among children and adults with gingivitis may perhaps help in assessing the subject’s risk to periodontitis. The low sample size is the main limitation of the present study. Further studies with large sample size might aid in close association of this genotype to clinical conditions of chronic marginal gingivitis.
§ Refers to Chi-square test; ¥ Unpaired t-test; & Fisher’s exact test; ** significant p value.