Allergic Ocular Diseases (AODs) like Atopic Keratoconjunctivitis (AKC) and Vernal Keratoconjunctivitis (VKC) are chronic forms of an ocular allergy that can cause severe visual complications [1–3]. AODs are IgE- and Th2- mediated allergic reaction with additional, not well-defined, perhaps nonspecific hypersensitivity responses [4]. The uncertain pathogenesis of AODs and the often ineffective anti allergic therapy has been a challenge for ophthalmologist [1].
Patient and parents should be instructed about the length of the disease and possible complications [1,5]. Drug therapy for AODs often comprise of topical anti-allergic agents and steroids [7]. However, efficacy of anti-allergic agents is poor and the use of ocular steroids are usually associated with risk of glaucoma, cataract and ocular hypertension [5,6]. Tacrolimus is 23-member cyclic macrolide lactone and derived from [streptomyces tsukubaensis] in 1984, was initially used as immunosuppressant in organ transplantation [7,8] and dispensed as formulation with castor oil, olive oil or dextrin [9–11]. Currently, it is available as ointment form for ophthalmic use.
This prospective observational study was designed to assess therapeutic effect of 0.1% Tacrolimus eye ointment in patients with severe AODs like Atopic keratoconjunctivitis and Vernal keratoconjuctivitis refractory to topical steroids and Cyclosporine medication.
Materials and Methods
This prospective observational study was conducted on 36 patients of AODs at ophthalmology department, Rajiv Gandhi Medical College from March 2015 to August 2015. Ethical committee approval was taken. Patients giving voluntary written informed consent for participation in study were included.
Inclusion Criteria
Diagnosed cases of severe AODs and moderate AODs not responding after three weeks of treatment with topical anti allergic and/or steroid medication in Rajiv Gandhi Medical College, Thane, Maharashtra, India.
Age group <40 years of both gender.
Exclusion Criteria
Ocular abnormalities (lid scarring, entropion, trichiasis, etc.).
Iris or anterior chamber pathology.
Glaucoma.
Contact lens wearer.
Corneal pathology (marginal ulcer, opacity, scar, bullous keratopathy, conjunctivochalasis, symblepharon, infection or tumour).
Pregnancy.
Non consenting patient.
Known hypersensitivity to Tacrolimus eye ointment.
These patients were treated with Tacrolimus ophthalmic eye ointment 0.1% (Talimus-Ajanta Pharmaceuticals, India) in affected eye twice daily for three months in addition to conventional treatment. Patients were observed for period of 6 months.
Six symptoms (viz. itching, discharge, lacrimation, photophobia, foreign body sensation and eye pain) were scored at initiation of treatment (Baseline) and at one month intervals, for six months. For any adverse reactions, patients were asked to contact telephonically or visit emergency department. Photographic criteria were used to grade (0=none, 1=mild, 2=moderate and 3=severe) each of the ten clinical signs.
Data sheet was maintained with above observations at every visit.
Patients who discontinued treatment within 1 month of treatment and lost to follow up before three months of treatment were eliminated from the study.
Age, gender, disease complications, previous medications and their side effects were the varying factors. The reduction in total signs and symptoms scores (from baseline) was used as the determinant of efficacy. [Table/Fig-1] Shows grading scales of clinical signs [12].
Grading scale of clinical signs [12].
| Signs | Score | Definition |
|---|
| Palpebral conjunctivaHyperaemia | 3 | Impossible to distinguish individual blood Vessels |
| 2 | Dilatation of many vessels |
| 1 | Dilatation of several vessels |
| 0 | None |
| Oedema | 3 | Diffuse oedema with opacity |
| 2 | Thinner diffuse oedema |
| 1 | Slight oedema |
| 0 | None |
| Follicles | 3 | 20 or more follicles |
| 2 | 10–19 follicles |
| 1 | 1–9 follicles |
| 0 | None |
| Papillae | 3 | Papillae size: 0.6 mm or more |
| 2 | Papillae size: 0.3–0.5 mm |
| 1 | Papillae size: 0.1–0.2 mm |
| 0 | None |
| Giant Papillae (papillaeSize≥1 mm) | 3 | Elevated papillae in 1/2 or more of the Upper Palpebral conjunctiva |
| 2 | Elevated papillae in <1/2 of the upper Palpebral conjunctiva |
| 1 | Flat papillae |
| 0 | None |
| Bulbar ConjunctivaHyperaemia | 3 | Diffuse dilated blood vessels over the entire bulbar conjunctiva |
| 2 | Dilatation of many vessels |
| 1 | Dilatation of several vessels |
| 0 | None |
| Oedema | 3 | Bullous oedema |
| 2 | Thinner diffuse oedema |
| 1 | Localized oedema |
| 0 | None |
| Limbus Trantas dot | 3 | 9 > dots |
| 2 | 5-8 dots |
| 1 | 1-4 dots |
| 0 | None |
| Swelling | 3 | Found in 2/3 or more of the limbal circumference |
| 2 | Found in 1/3 to <2/3 of the limbal circumference |
| 1 | Found in <1/3 of the limbal circumference |
| 0 | None |
| Corneal Epithelial signs | 3 | Shield ulcer or corneal erosion |
| 2 | Exfoliation superficial punctate keratitis |
| 1 | Superficial punctate keratitis |
| 0 | None |
Statistical Analysis
Data were coded and entered in Microsoft Excel 2010 and analysed using SPSS (Statistical Package for Social Sciences) version 20. Data were presented as mean±SD. Statistical significance was defined as a two sided p-value <0.05. Wilcoxon signed rank test was used to compare pre and post treatment total scores.
Results
Of the 41 patients initially included in this study, 4 patients were eliminated from the study since they did not follow-up for 3 months. One patient complained of severe burning sensation after instillation of Tacrolimus eye ointment over 2 weeks. After 2 weeks, patient stopped the treatment and refused to restart the same on next visit. This patient was associated with atopic dermatitis. Thus, 36 patients were included in analysis of study and their data were analysed. The percentage of male patients was 69.44%. Mean patient age was 9.3±4.3 years and mean duration of AODs 3.1±1.8 years. Allergic rhinitis and asthma were also present in 16.66% and 11.11% of patients, respectively. Of all patients analysed, 77.77% had previously used anti-allergic ophthalmic solutions such as antihistamine and mast cell stabiliser, 72.22% had previously used steroid ophthalmic solutions, and 11.11% had previously used Cyclosporine eye drop 0.1%.
Change in signs and symptoms with topical Tacrolimus eye drop use
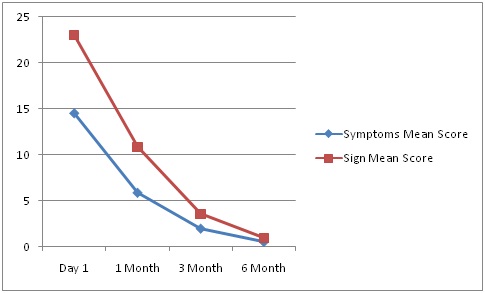
The total score of the 10 clinical signs (range 0–30) and 6 clinical symptoms (range 0–18) significantly decreased from baseline to 1 month in follow-up after beginning Tacrolimus eye ointment treatment (p<0.0001, Wilcoxon signed rank test). The mean total score of clinical symptoms was 14.50±2.46 at the start of treatment and decreased to 0.56±0.69 at the last observation (mean change from baseline=−13.96). The total clinical sign score decreased from 23.00±2.29 at baseline to 1.00±1.17 at the last observation (mean change from baseline=−22.0) [Table/Fig-2,3,4,5,6,7,8,9,10,11,12 and 13].
Changes from baseline in symptom scores in patients.
| Descriptive Statistics |
|---|
| 6 Symptoms score (0-18) | N | Minimum | Maximum | Mean | Std. Deviation |
|---|
| Day one | 36 | 11 | 17 | 14.50 | 2.467 |
| One month | 36 | 3 | 9 | 5.89 | 1.879 |
| Three months | 36 | 0 | 5 | 2.00 | 1.656 |
| Six months | 36 | 0 | 2 | 0.56 | 0.695 |
| Valid N (list wise) | 36 | | | | |
Statistical analysis of symptoms score.
| Test Statistics |
|---|
| Test | One month - 6 Symptoms score (0-18) Day one |
|---|
| Z | -5.267b |
| Asymp. Sig. (2-tailed) | 0.0001 |
| a. Wilcoxon Signed Ranks Test |
| b. Based on positive ranks. |
a Statistically Significant decreased in symptoms score from baseline to 1 month (p<0.0001) using Wilcoxon signed rank test
Changes from baseline in sign scores in patients.
| Descriptive Statistics |
|---|
| 10 signs score (0-30) | N | Minimum | Maximum | Mean | Std. Deviation |
|---|
| Day one | 36 | 19 | 25 | 23.00 | 2.293 |
| One month | 36 | 9 | 13 | 10.89 | 1.469 |
| Three months | 36 | 0 | 7 | 3.56 | 2.833 |
| Six months | 36 | 0 | 3 | 1.00 | 1.171 |
| Valid N (listwise) | 36 | | | | |
Statistical analysis of sign score.
| Test Statistics |
|---|
| Test | One month- 10 signs score (0-30) Day1 |
|---|
| Z | -5.297b |
| Asymp. Sig. (2-tailed) | 0.0001 |
| a. Wilcoxon Signed Ranks Test |
| b. Based on positive ranks. |
a Statistically Significant decreased in signs score from baseline to 1 month (p<0.0001) using Wilcoxon signed rank test
Changes from baseline in total sign and symptom scores in patients on Tacrolimus.
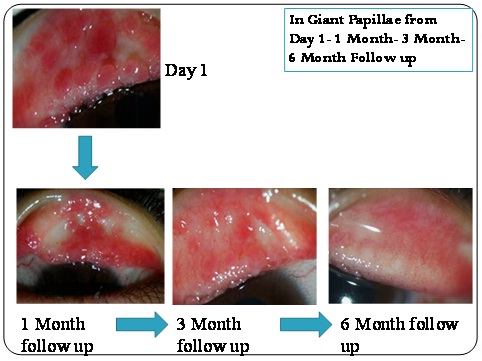
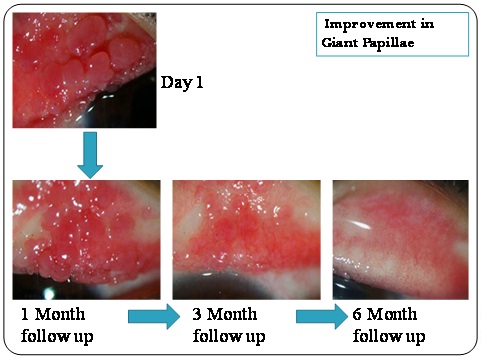
Decrease in Giant Papillae from baseline till 6 months in a patient on Tacrolimus.
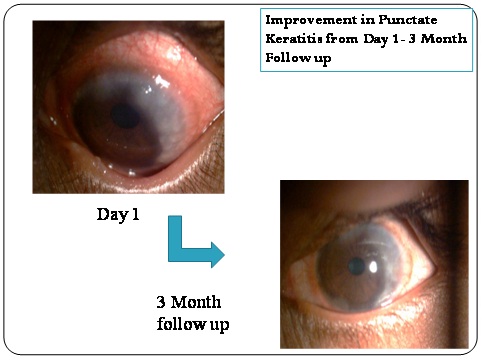
Improvement of punctate keratitis from baseline till 3 months follow-up in a patient on Tacrolimus.
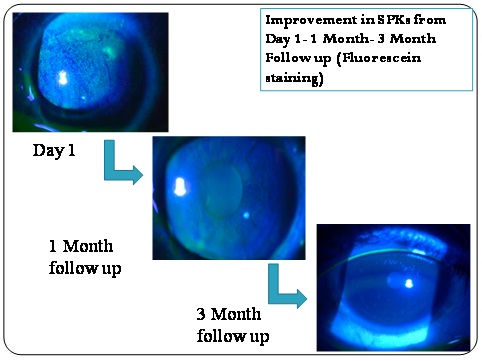
Improvement of punctate keratitis with flourescein staining from baseline till 6 months follow up in a patient on Tacrolimus.
Giant Papillae from baseline till 6 months in patients on tacrolimus showing a decline.
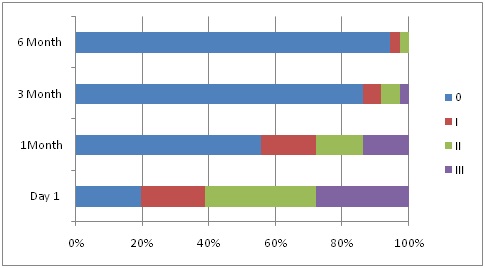
Decrease in Giant Papillae from baseline till 6 months in a patient on Tacrolimus.
HT dots from baseline till 6 months in patients on tacrolimus showing a decline.
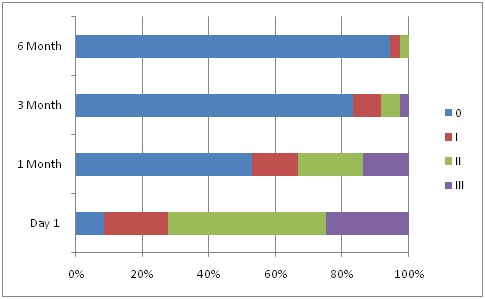
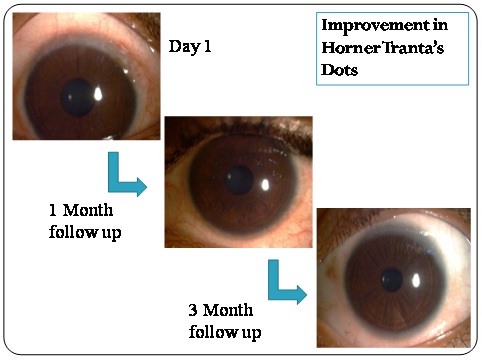
Decrease in HT dots from baseline till 6 months in a patient on Tacrolimus.
Proportion of patients on steroid therapy
We assessed the possible steroid-sparing effect of Tacrolimus eye ointment. The percentage of patients (n=36 patients at baseline) using each type of steroid ophthalmic solution progressively decreased during the Tacrolimus eye ointment administration period. As shown in [Table/Fig-14,15], 88.88% of patients were successfully weaned off topical steroids 6 months into Tacrolimus treatment.
Percentage of patients successfully weaned off topical steroids 6 months into Tacrolimus treatment.
| Drugs | Day one | 1 Month | 3 Months | 6 Months |
|---|
| Steroids | 36 | 30(83.33%) | 10(27.77%) | 4(11.11%) |
| Steroid free | 0 | 6(16.66) | 26(72.23%) | 32(88.88%) |
Patients with adverse reactions.
| Adverse drug reaction | Number of patients | Percentage % |
|---|
| Transient burning sensations | 13 | 36.11% |
| Severe burning sensation | 1 patient discontinued after 2 weeks | 2.7% |
| Eye irritation | 03 | 8.10% |
| Eye pain | 02 | 5.40% |
| Hordeolum | 00 | - |
| Conjunctival hyperemia | 00 | - |
| Eye pruritis | 00 | - |
| Foreign body sensation | 03 | 8.10% |
| Eye discharge | 00 | - |
| Lacrimation | 03 | 8.10% |
Adverse reactions
Adverse reactions were noted in 16 (44.44%) of 36 patients analysed. The only major adverse reaction was a transient burning sensation upon topical medication (13 cases, 36.11%). Corneal infections, including bacterial keratitis or herpetic keratitis were not observed in our study.
Score changes in patients unresponsive to topical Cyclosporine 0.1%
Four patients previously treated with 0.1% Cyclosporine eye drops for a minimum two months, had Giant Papillae or corneal epithelial disorder scores >2 when Tacrolimus therapy was started. After one month of Tacrolimus treatment, the total clinical sign score in these four patients had decreased from 24.33±2.21 to 1.66±1.02 (change=−22.67), while the total clinical symptoms score reduced from 16.33±2.16 to 1.00±0.79 (change=−15.33) [Table/Fig-16]. The reduction in these score was significant (p<0.001, Wilcoxon signed rank test)
Changes from baseline in total sign and symptom scores in patients refractory Cyclosporine ophthalmic therapy.
| Day 1 | 1 Month | 3 Months | 6 Months |
|---|
| MEAN SCORE OF SYMPTOMS (0-18) | 16.33 ± 2.16 | 8.0± 1.537 | 3.33 ± 1.25 | 1.0 ± 0.79 |
| MEAN SCORE OF SIGNS (0-30) | 24.33 ± 2.21 | 11.6 ± 1.29 | 5.33 ± 2.35 | 1.66 ± 1.02 |
Discussion
Understanding and treating AODs has been a challenge for ophthalmologists [1,2]. Variety of medications have been used which include anti-histamines, mast-cell stabilisers, and non-steroidal anti-inflammatory drugs, steroids, immunosuppressant as topical preparations [1,2].
Tacrolimus is a potent immunosuppressive agent, which inhibits several immune reactions involved in the pathogenesis of AODs [7,8,13]. Initially Tacrolimus was used in the form of oral preparation in refractory severe AODs [14]. In few studies, they have used either a commercially available 0.03–0.1% ‘skin’ ointment which was applied to eyelids or fornix, or own-made 0.02–0.1% ointment for the topical use in eyes [15,16]. Tacrolimus was later formulated to be an isotonic aqueous suspension using polyvinyl alcohol as dispersive agent [17]. Now Tacrolimus is available in eye drop and eye ointment form [18]. Various studies have reported that Tacrolimus is 100 times more potent than Cyclosporine inhibit calcineurine activity which play important role in Allergic Ocular diseases physiology [12,19].
In our study, we used topical Tacrolimus eye ointment 0.1% concentration. Till date, a single Indian study of efficacy of Tacrolimus 0.03% eye ointment in AODs has been reported from India [20]. So this study was carried out to assess the safety and efficacy of 0.1% Tacrolimus eye ointment in patients with Allergic Ocular Diseases (AODs) in Indian population.
All 36 patients included in study showed statistically significant improvement in symptoms and signs by the end of 1 month treatment (p<0.0001). In our study mean score of symptoms base line (14.50±2.46) was improved at the end of 6 month (0.56±0.69) which was statistically significant (p<0.0001). Similarly, Fukushima et al., found improvement of score base line 8.1±4.5 to 1.8±2.8 at last observation [12]. Also, Kheirkhah et al., noticed change in base line score 10.0 to 0.80 at end of 3 month [17]. Itching and lacrimation showed rapid and dramatic improvement (>65% improvement in symptoms) at 1 month of treatment. Rest of symptoms also showed remarkable improvement by the end of 3 month. Kheirkhah et al., also observed that itching was mostly relieved in 80% patients by 1 month after starting Tacrolimus eye drop [17]. Also, studies by Miyazaki et al., and Ohashi et al., have reported marked improvement in symptoms at the end of 1month [18,21].
In our study, there was clinical signs base line score (23.0±2.29) improvement at the end of 6 months to score (1.0±1.17) which was statistically significant (p<0.0001). Similarly, Fukushima et al., found mean baseline score 15.3±5.0 improved to 5.9 ±4.6 at last observation which was statistically significant (p<0.0001) [12]. Also, the study by Kheirkhah et al., showed statistically significant improvement in sign score from baseline to 3 month follow-up [17]. Similarly Hazarika and Singh reported day 1 mean score 9.6±3.27 improved to 1.35±1.19 at day 7 which was statistically significant (p<0.0001) [20]. Ohashi et al., observed the baseline and final follow up score difference -5.6±5.1 which is statistically significant [21]. In our study, we found Conjunctival Hyperaemia showed marked improvement by the end of 1 month from baseline (100%) to end of 1 month (40%). Similarly, Kheirkhah et al., noticed Conjunctival Hyperaemia was the first sign to show improvement with marked reduction of hyperaemia within 1 month of treatment [17]. In our study, Giant Papillae score was improved from base line (80.55%) to end of 6 month (13.88%) which significant (p<0.001). Similarly, Fukushima et al., noticed improvement in Giant Papillae from baseline 87.2% to 15.8% at last follow up which was statistically Significant (p<0.001) [12]. Also, Kheirkhah et al., and Ohashi et al., observed significant reduction in Giant Papillae at end of 1 month treatment [17,21].
In our study, 4 patients were refractory to topical Cyclosporine treatment and they showed good response to Tacrolimus eye ointment by showing significant change in symptoms and signs score. Base line Symptoms score (16.33±2.16) and Signs score (24.33±2.21) of these patients improved to score (1±0.79) and (1.66±1.02), respectively with Tacrolimus eye ointment which is statistically significant (p<0.001). Fukushima et al., also noted base line score of symptoms score16.8±4.7 and signs score 9.1±4.4 significant improvement (p<0.001) to 6.7±4.9 and 1.9±3 respectively [12].
In the present study, steroids were used concomitantly. Our data shows 88.88% of patients successfully weaned off from topical steroids. Thus, Tacrolimus eye ointment has steroid sparing and replacing effect. Fukushima et al., also assessed steroid sparing effect of Tacrolimus and found 53.4% patients successfully weaned off topical steroids [12].
In our study, most commonly observed complaints were transient burning sensation in 13 patients and eye irritation in 3 patients. No corneal complications were noted in our study with Tacrolimus eye ointment. A study by Hazarika and Singh observed transient foreign body sensation in 39% patients, transient burning in 34% patients and transient blurring of vision in 26% [20]. Ohashi et al., reported 12 cases with transient burning sensation but no other adverse effect noted with topical Tacrolimus use [21]. Fukushima et al., reported complications like transient burning sensation in 46 patients, corneal involvement (Bacterial ulcer and herpetic keratitis) seen in 10 patients, and Hordeolum noted in 8 patients [12]. A study by Kheirkhah et al., did not find any adverse drug side effects with 0.005% Tacrolimus eye drops [17].
Conclusion
Topical 0.1% Tacrolimus eye ointment is a well tolerated and effective in management of patients with AODs. It has topical steroid sparing and replacing effect in patients with AODs which avoid the vision threatening complications associated with topical steroid therapy. Also, the patients not responding to topical Cyclosporine showed response to Tacrolimus eye ointment.