Red Lentil Extract: Neuroprotective Effects on Perphenazine Induced Catatonia in Rats
Gholamreza Houshmand1, Shahram Tarahomi2, Ardeshir Arzi3, Mehdi Goudarzi4, Mohammad Bahadoram5, Mohammadreza Rashidi-Nooshabadi6
1 Phd Condidate, Department of Pharmacology and Toxicology, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; Medical Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2 Lecturer, Department of Neurology, Abadan University of Medical Sciences, Abadan, Iran.
3 Professor, Department of Pharmacology and Toxicology, School of Pharmacy, Physiology Research Center, Jundishapur University of Medical Sciences, Ahvaz, Iran.
4 Phd Condidate, Department of Pharmacology and Toxicology, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
5 General Practitioner, Medical Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
6 Phd Condidate, Department of Pharmacology and Toxicology, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Gholamreza Houshmand, Department of Pharmacology and Toxicology, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
E-mail: houshmand.g@ajums.ac.ir
Introduction
Parkinsonism is a neurodegenerative disease that is defined by certain symptoms such as muscle rigidity, impaired movement, catatonia, tremor and disorientation of body.
Aim
The aim was to investigate the effect of red lentil extract on perphenazine-induced Catatonia in model of rat.
Materials and Methods
This experimental study was done on 48 male albino rats (weight 180–200g) of the Sprague-Dawley strain. Animals were randomly divided into six groups and were pre-treated with a single dose of red lentil extract (200, 400, 800 and 1000 mg/kg), most effective dose of bromocriptine (30mg/kg) and normal saline (5ml/kg) via intraperitoneal (IP) route. perphenazine (5 mg/kg) was after 30 minutes, administered (IP) to induce catatonia. The scoring method of Morpurgo was used to determine the muscular rigidity of animals.
Results
The results showed that the 200mg/kg red lentil extract treated group had no significant reduction in catatonic responses after perphenazine administration in comparison with control group while the groups that received 800 and 1000mg/kg of red lentil extract showed significant difference (p<0.05) at all the time points.
Conclusion
The results revealed that hydroalcoholic extract of red lentil has protective effect on Catatonia induced by perphenazine in rats. So this extract may be probably beneficial for catatonia in Parkinsonism.
Parkinsonian Disorders, Muscle Rigidity, Antioxidants, Leguminoseae
Introduction
Parkinson’s Disease (PD) is the most common neurodegenerative disorder- which affects 1% of the population by the age of 65 and 4–5% of the population by the age of 85 [1]. The loss of dopaminergic neurons in the substantia nigra pars compacta is considered as the major cause of PD [2]. Parkinson disorder can be idiopathic, that is the classically described by Parkinson, or caused by other factors, among them are drug-induced; in this case, it is termed pseudoparkinsonism. The latter has been used in experimental animal models for studying the effects of various pharmacological interventions for assessment of effectiveness of drugs used in the treatment of this disorder [3,4].
The symptoms of the disease include limbs and trunk rigidity; jaw, face, hands, arms and legs tremor; bradykinesia and postural instability [1]. A variety of medication options and medical strategies has been put forward as a means of PD treatment [5].
Many studies suggests that, oxidative stress on substantia nigra as a causative factor of Parkinson’s disease [6–8]. Hydroxyl radicals, a form of reactive oxygen species, have been proposed to be responsible for mediating the dopamine neuronal cell death in the nigrostriatal system in PD [8,9]. If oxidative stress is relevant in the pathogenesis of PD, use of antioxidant substances in the diet could theoretically influence the risk for this disease. Natural phenolic antioxidants has the potential to scavenge Reactive Oxygen and Nitrogen Species (RONS) and may prevent the onset of oxidative diseases in the body [10].
Antioxidants mainly act by:
Inhibiting the NADPH oxidase and reduce NADPH oxidase-mediated generation of reactive oxygen species.
Balance NO production from different NO synthase isoforms.
Reducing neuroiniflammation via attenuation of the release of cytokines and down regulation of the pro-inflammatory transcription factors.
Modulating signaling pathways such as mitogen-activated protein kinase cascade and cAMP response element-binding protein are responsible for the neuroprotective actions of different natural polyphenols [11–14].
The lentil plant, Lens culinaris L, is a member of the Leguminoseae family. Leguminous seeds come from plant foods, that are generally rich in phenolic compounds, including condensed tannins [15,16]. Some researchers have investigated the antioxidant activity of phenolic compounds that are extracted from leguminous seeds by using several in-vitro chemical assays [17–19]. In their study, Xu and Chang found that lentils had the highest antioxidant volume when measured as 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging volume compared to yellow pea, green pea and chickpea [20]. According to the United States Department of Agriculture (USDA), Oxygen Radical Absorbing Capacity (ORAC) values 2007, lentils had a higher ORAC value than most of the common fruits and vegetables including apples, blackberries, figs, cherries, pears, oranges, garlic, cabbage and almonds [21]. Lentil seeds are used now-a-days in the folk medicine of many ethnicities to treat different illnesses. They are used orally to treat diabetes, topically as a water paste to treat skin infections and for the treatment of burns, after being roasted, milled and applied directly to affected areas [22,23].
In the current study, we used an experimental model proposed earlier by Morpurgo in 1962, which is based on the perphenazine-induced catatonic reaction [24]. In this study we aimed to compare the efficiency of bromocriptine (Dopamine agonist) and red lentil extract in the prevention of perphenazine-induced pseudoparkinsonism in rats.
Materials and Methods
Extract Preparation
Dry seeds of red lentil were collected from Khuzestan region, Iran in September 2013. The seeds were identified at the Herbarium of Department of Pharmacognosy, School of Pharmacy, Ahvaz, Iran. 500 g red lentil seeds was crushed into small pieces and soaked in a 70% aqueous-ethanol solution in a large container for 3 days with regular shaking. The extract was filtered through a clean cotton cloth and then dried using a rotary evaporator at 40°C.
Animals
Forty-eight adult male albino rats (weight 180–200g) of the Sprague-Dawley strain were obtained from the animal house of Ahvaz Jundishapur University of Medical Science, Iran. Animals were kept under standard condition in polycarbonate cages (12h light/dark cycle, Temperature 23±2°C, relative humidity of 45% to 55%). They had access to rat’s chow and water ad libitum. The animals were given one-week time to acclimatize to the environment before the commencement of experiment. Animal studies were followed according to Institute Animal Ethics Committee (IAEC) regulations approved by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (Reg. No. PRC- 9303) and conducted humanely.
Experimental Design
The animals were randomly divided into six groups (n=8) and were pre-treated with a single dose of red lentil extract (RLE) (200, 400, 800 and1000 mg/kg), most effective dose of bromocriptine (30mg/kg) and normal saline (5ml/kg) via intrapritoneal. After 30 minutes, all animals received an IP injection of 5mg/kg perphenazine hydrochloride and relative muscular rigidity was determined for given minutes after injection as follows; 20, 40, 60, 90, 120, 180 and 240. The method of Morpurgo was used to determine muscular rigidity [24]. We closely observed the development of catatonia and the following scores were achieved: Stage 1, free movement of rat on the table, score allocated = 0; Stage 2, rat moves only by touch or push, score allocated = 0.5; Stage 3, the rat’s front paws are alternately placed on a high block of 3cm height. Failure to correct the posture in 10 seconds, score allocated = 0.5 for each paw with a total Score of 1 for this stage. Stage 4, the rat front paws are alternately placed on a 9cm high block. Failure to correct the posture in 10 seconds, score allocated = 1 for each paw with total score of 2 for this stage. Therefore, the maximum possible score for a single rat, would be 3.5 displaying total catatonia. Lower score suggests a lower degree of catatonia.
Statistical Analysis
Results of treatment effects were statistically analysed using Kruskal-Wallis non parametric test. The p-value less than 0.05 were considered the level of significant difference in all tests.
Results
The mean of the scoring for all groups under investigation are reported and summarized in [Table/Fig-1]. The results showed 200 mg/kg RLE treated group had no significant reduction in catatonic responses in all of minutes after perphenazine administration in comparison with control group (normal saline).
Mean ± the Standard Error of the Mean (SEM) perphenazine-induced catatonic reaction scores at various intervals in pretreatment with Normal saline, red lentil extract or Bromocriptine groups in rat.
| Minutes Following Administration Of Perphenazine |
|---|
| Group | 20 | 40 | 60 | 90 | 120 | 180 | 240 |
|---|
| Normal saline | 1.53±0.10 | 2.05±0.15 | 3.14±0.24 | 3.5±0.11 | 3.5±0.10 | 3.5±0 | 3.5±0 |
| RLE 200 | 1.17±0.16 | 1.67±0.12 | 2.5±0.20 | 3.2±0.18 | 3.3±0.08 | 3.5±0 | 3.5±0 |
| RLE 400 | 0.81±0.10* | 1.05±0.13* | 1.9±0.23* | 2.25±0.09* | 2.7±0.10* | 3.02±0.03* | 3.35±0 |
| RLE 800 | 0.61±0.07* | 0.86±0.10* | 1.49±0.11* | 1.86±0.13* | 1.89±0.09*# | 2.4±0.05*# | 2.99±0.01* |
| RLE 1000 | 0.59±0.09* | 0.72±0.09* | 1.38±0.15* | 1.75±0.09* | 1.94±0.11*# | 2.3±0.08*# | 2.84±0.03* |
| Bromocriptine | 0.14±0.03* | 0.32±0.04* | 0.74±0.08* | 1.02±0.10* | 1.62±0.07* | 2.1±0.12* | 2.3±0.07* |
*Significantly different from normal saline treated group. p <0 .05
# Non-Significantly different from bromocriptine treated group. p <0 .05
While the groups that received 800 and 1000mg/kg of RLE in comparison with control group showed significant difference (p<0.05) in all the period of the experiment (240 minutes). The group which received 400mg/kg of RLE in comparison with control group showed significant difference (p<0.05) in all specified times of trial except 240 min [Table/Fig-2].
The effect of different doses of Red Lentil Extract (RLE) on perphenazine (Perph)-induced rigidity in rats.
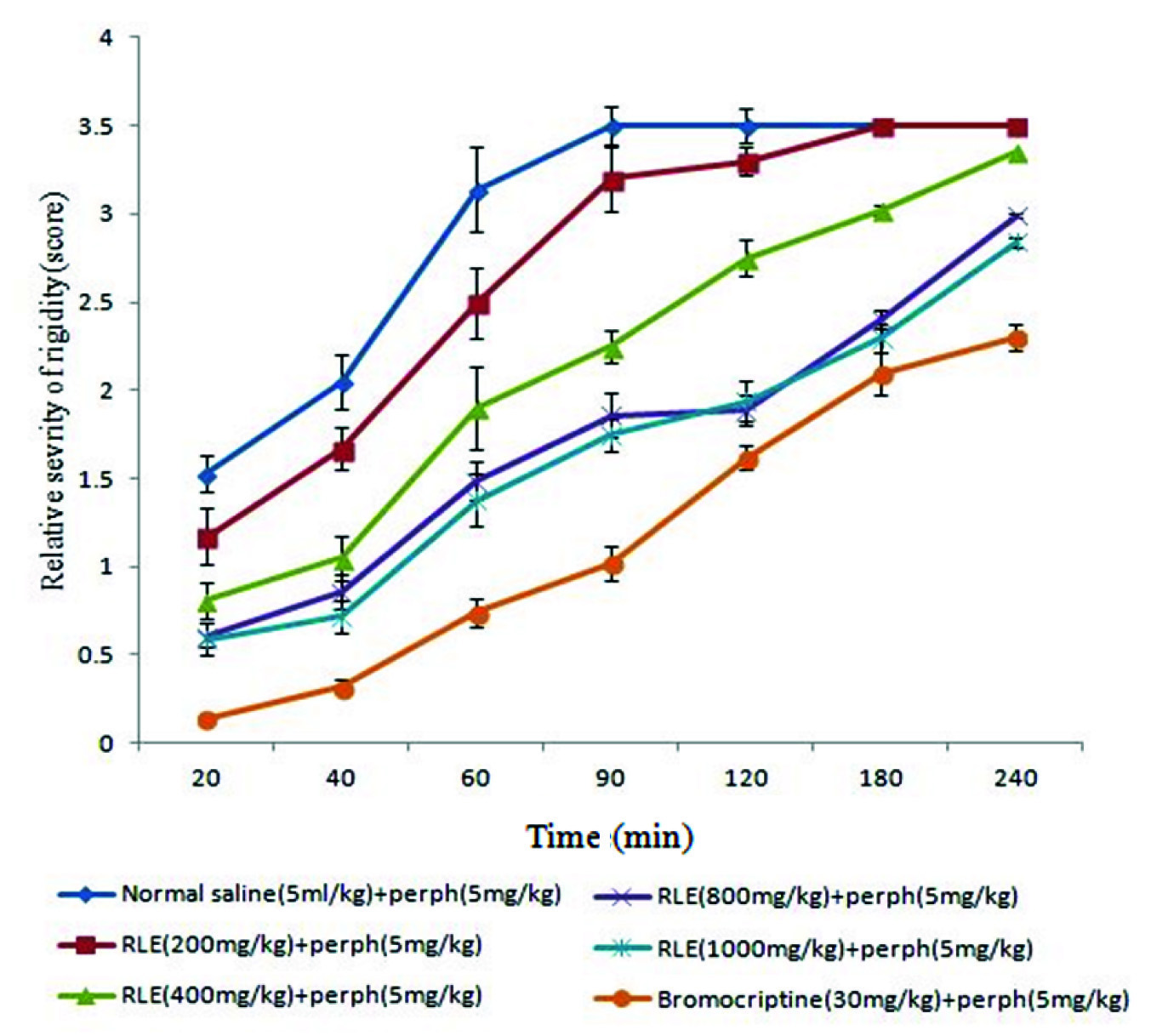
There was no significant difference in catatonic responses between dose of 800 and 1000 mg/kg of RLE in all the period of the experiment (240 minutes) after perphenazine administration.
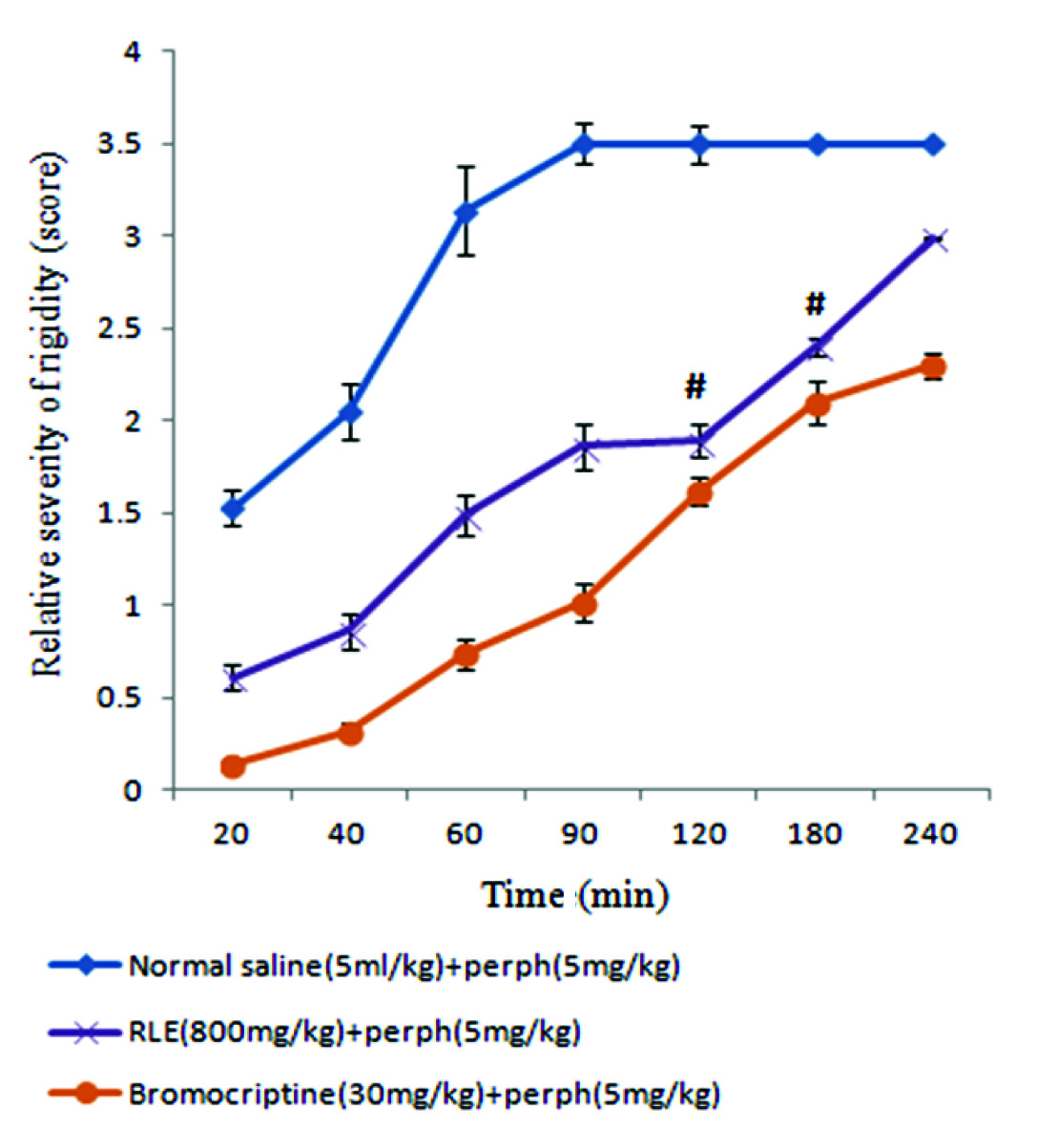
There was no significant difference in catatonic responses between doses of 800 and 1000mg/kg of RLE with effective dose of bromocriptine (30mg/kg) at minutes 120 and 180 after perphenazine treatment [Table/Fig-3].
Comparison of the effect of red lentil extract and bromocriptine on perphenazine-induced rigidity in rats.
# Non-Significantly different from bromocriptine treated group. P <0 .05
RLE: red lentil extract; Perph: perphenazine
Discussion
Therapeutic approaches to stop the progression of PD are yet to be developed since the etiology of the disease remains unknown. It is suggested that the pathogenesis of Parkinson’s may involve oxidative and nitrative stress, excitotoxicity, inflammation, altered proteolysis mitochondrial dysfunction, interrelated events that lead to neuron death by apoptosis [4].
Severe oxidative stress progressively leads to cell dysfunction and ultimately cell death. Any disruption in the balance between free radical production and antioxidant processes can lead to oxidative stress [6,7]. Epidemiological data suggest that antioxidants may have a beneficial effect on many age-related disease including neurodegenerative diseases [25]. Pulses are increasingly gaining more interest in the fields of developing healthy and functional foods these days. Lentils had a kind of pulse, gained much interest with respect to its unique nutritional and functional characteristics [23]. Lens culinaris L, a lentil plant, belongs to the Leguminoseae family and makes up what is considered to be one of the most important traditional dietary components. Among polyphenols, quercetin diglycoside, catechin, digallate procyanidin, and p-hydroxybenzoic, tannins and tannin-related compounds are principal in lentils, and mainly concentrated in the its testa [26].
After the apoptosis is triggered by neurotoxic species, they get inhibited by flavonoids. The flavonoids are famous for promoting neuronal survival and synaptic plasticity. They do these through interaction with critical protein and lipid kinase signaling cascades in the brain. The flavonoids generate positive effects on the vascular system and are likely to make changes in the cerebrovascular blood flow, possibly leading to angiogenesis, neurogenesis and changes in neuronal morphology. These mechanisms could diminish the chance of neurodegeneration and to block or reverse agedependent loses in cognitive performance by the consumption of flvonoid-rich foods throughout life [27].
Zhang et al., demonstrated that quercetin could prevent 6-OHDA-stimulated dopaminergic neuron damage and 6-OHDA-induced PC12 cell apoptosis in zebrafish [28]. In the current study, Quercetin could inhibit inducible nitric oxide synthase (iNOS) over-expression and NO over-production in PC12 cells and could down-regulate the over-expression of pro-inflammatory genes (e.g. IL-1β, TNF-· and COX-2) in zebrafish, so suggests that these genes have important role in the neuroprotective effect of quercetin.
According to our study and review of the literature, we suggest that red lentils extract with a high quantity of quercetin could modulate expression of pro-inflammatory cytokines such as TNFα, IL-1β and also suppress neuron appoptosis and prevent degeneration.
Lentils showed the highest total antioxidant capacity among tested pulses measured by ferric reducing antioxidant power and total radical-trapping antioxidant parameter measures, but came second to broad beans by Trolox equivalent antioxidant capacity measure. On the other hand, flavonoids, such as glycosides of flavonols and flavones, are mainly present in the seed coat of lentils [15,29].
Conclusion
The results from this study demonstrate that red lentil was effective in reducing acute perphenazine-induced catatonic muscular rigidity in rats. This effect was dose-dependent.
*Significantly different from normal saline treated group. p <0 .05
# Non-Significantly different from bromocriptine treated group. p <0 .05
[1]. Pahwa R, Lyons KE, Koller W, Handbook of Parkinson’s disease 2012 CRC Press [Google Scholar]
[2]. Hirsch EC, Jenner P, Przedborski S, Pathogenesis of parkinson’s diseaseMovement Disorders 2013 28(1):24-30. [Google Scholar]
[3]. Przedborski S, Animal models of PDParkinsonism & Related Disorders 2012 18:S164 [Google Scholar]
[4]. Schapira AH, Jenner P, Aetiology and pathogenesis of Parkinson’s diseaseMovement Disorders 2011 26(6):1049-55. [Google Scholar]
[5]. Singh N, Pillay V, Choonara YE, Advances in the treatment of Parkinson’s diseaseProgress in neurobiology 2007 81(1):29-44. [Google Scholar]
[6]. Hwang O, Role of oxidative stress in Parkinson’s diseaseExperimental Neurobiology 2013 22(1):11-17. [Google Scholar]
[7]. Jenner P, Oxidative stress in Parkinson’s diseaseAnnals of Neurology 2003 53(S3):S26-38. [Google Scholar]
[8]. Jenner P, Oxidative mechanisms in nigral cell death in Parkinson’s diseaseMovement disorders: official journal of the Movement Disorder Society 1997 13:24-34. [Google Scholar]
[9]. Jenner P, Olanow CW, Oxidative stress and the pathogenesis of Parkinson’s diseaseNeurology 1996 47(6 Suppl 3):161S-70S. [Google Scholar]
[10]. Halliwell B, Gutteridge J, Cross C, Free radicals, antioxidants, and human disease: where are we now?The Journal of Laboratory and Clinical Medicine 1992 119(6):598 [Google Scholar]
[11]. Aquilano K, Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenolsNeurochemical Research 2008 33(12):2416-26. [Google Scholar]
[12]. Campos-Esparza MR, Sanchez-Gomez MV, Matute C, Molecular mechanisms of neuroprotection by two natural antioxidant polyphenolsCell calcium 2009 45(4):358-68. [Google Scholar]
[13]. Vafeiadou K, Vauzour D, Spencer J, Neuroinflammation and its modulation by flavonoidsEndocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 2007 7(3):211-24. [Google Scholar]
[14]. Kovacsova M, Neuroprotective mechanisms of natural polyphenolic compoundsAct Nerv Super Rediviva 2010 52(3):181-86. [Google Scholar]
[15]. Zou Y, Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractionsJournal of Agricultural and Food Chemistry 2011 59(6):2268-76. [Google Scholar]
[16]. Amarowicz R, Pegg RB, Legumes as a source of natural antioxidantsEuropean Journal of Lipid Science and Technology 2008 110(10):865-78. [Google Scholar]
[17]. Madhujith T, Amarowicz R, Shahidi F, Phenolic antioxidants in beans and their effects on inhibition of radical-induced DNA damageJournal of the American Oil Chemists’ Society 2004 81(7):691-96. [Google Scholar]
[18]. Amarowicz R, Troszyska A, Antioxidant activity of extract of pea and its fractions of low molecular phenolics and tanninsPol J Food Nutr Sci 2003 12(53):10-15. [Google Scholar]
[19]. Amarowicz R, Troszyńska A, Pegg R, Antioxidative and radical scavenging effects of phenolics from Vicia sativumFitoterapia 2008 79(2):121-22. [Google Scholar]
[20]. Xu B, Chang S, A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solventsJournal of Food Science 2007 72(2):S159-66. [Google Scholar]
[21]. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2. Retreivable from http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/ORAC/ORAC_R2.pdf. (accessed October 2010) [Google Scholar]
[22]. Deshpande S, Food legumes in human nutrition: a personal perspectiveCritical Reviews in Food Science & Nutrition 1992 32(4):333-63. [Google Scholar]
[23]. Takruri HR, Issa AY, Role of lentils (Lens culinaris L.) in human health and nutrition: a reviewMediterranean Journal of Nutrition and Metabolism 2013 6(1):3-16. [Google Scholar]
[24]. Morpurgo C, Effects of antiparkinson drugs on a phenothiazine-induced catatonic reactionArchives Internationales de Pharmacodynamie et de Thérapie 1962 137:84 [Google Scholar]
[25]. Jenner P, Oxidative stress in Parkinson’s disease and other neurodegenerative disordersPathologie-Biologie 1996 44(1):57-64. [Google Scholar]
[26]. Boudjou S, Phenolics content and antioxidant and anti-inflammatory activities of legume fractionsFood chemistry 2013 138(2):1543-50. [Google Scholar]
[27]. Vauzour D, The neuroprotective potential of flavonoids: a multiplicity of effectsGenes & Nutrition 2008 3(3-4):115-26. [Google Scholar]
[28]. Zhang ZJ, Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafishInternational Journal of Molecular Medicine 2011 27(2):195 [Google Scholar]
[29]. Amarowicz R, Antioxidant activity of a red lentil extract and its fractionsInternational Journal of Molecular Sciences 2009 10(12):5513-27. [Google Scholar]