Prospective Randomized Study of Oral Diazepam and Baclofen on Spasticity in Cerebral Palsy
Vinay Goyal1, Nonica Laisram2, Ranjan Kumar Wadhwa3, Shashank Yashwant Kothari4
1 Formerly, Senior Resident, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjang Hospital, New Delhi, India.
2 Professor and Head of Department, Department of Physical Medicine and Rehabilitation, VMMC and Safdarjang Hospital, New Delhi, India.
3 SAG Officer, Department of Physical Medicine and Rehabilitation, VMMC & Safdarjang Hospital, New Delhi, India.
4 Retd. Professor, Department of Physical Medicine and Rehabilitation, VMMC & Safdarjang Hospital, New Delh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vinay Goyal, Flat No 6 03, Building No 10, KH -4, Celebrations, Sector 16, Kharghar, Navi Mumbai, Maharashtra-410210, India.
E-mail: vinaygoyal80@gmail.com
Introduction
Spastic cerebral palsy (CP) is the most common form of CP. Diazepam and Baclofen are the most commonly used oral drugs to manage spasticity. Study was designed to evaluate and compare their effects and safety in CP children.
Aim
Study was aimed to assess and compare outcome of oral Diazepam and Baclofen in spastic cerebral palsy children in terms of extent of reduction of spasticity and side effects profile.
Materials and Methods
Randomized prospective follow-up study was done for one year after giving Diazepam and Baclofen in weekly incremental doses upto recommended maximum dose to 60 children for three months. Two primary outcome measures were spasticity reduction and adverse effect profile. Spasticity reduction was measured by Modified Ashworth’s Scale (MAS) and Range of Motion improvement (ROM).
Results
After random allocation, there was no baseline difference between groups. Mean MAS score improved from 1.96±0.4 at baseline to 1.63±0.40 and 1.41± 0.36 at 1 month and 3 months for Diazepam and from 1.84±0.64 to 1.57±0.59 and 1.31± 0.48 respectively for Baclofen. Within the group reduction was significant with p-value = 0.0001. Intergroup comparison showed no statistically significant difference with p-value of 0.48 and 0.22 at 1 and 3 months. Baseline ROM showed significant improvement at 1 and 3 months with p value of 0.004 and 0.001 for Diazepam and 0.01 and 0.000 for Baclofen respectively with no statistically significant difference among two groups. Drowsiness was most common observed side effect in both the groups.
Conclusion
Patients showed significant improvement in spasticity as measured by Mean MAS score and range of motion in Diazepam as well as Baclofen group. Both drugs were found safe for use in children. Study couldn’t establish any difference between the two drugs. However studies with bigger sample size and longer follow- up assessing functional improvement in patients will be required in near future.
Childhood disabilities, Functional improvement, Modified ashworth’s scale
Introduction
Cerebral Palsy (CP) is defined as a group of permanent disorders of development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of CP are often accompanied by disturbances of sensation, cognition, communication, perception, behavior, seizure disorders and by secondary musculoskeletal problems [1]. Cerebral palsy incidence is 1.5-2.5 per 1000 live births [2,3] and contributes as the most common cause of the chronic childhood disabilities [4]. Most common type of CP is spastic type contributing 70 - 80 % of all cases [5]. Spasticity can cause discomfort, stiffness, pain and difficulty in performing physical activities such as washing, dressing, transfers, walking and picking up objects [6].
Spasticity can be managed by exercise therapy, oral medications, tone inhibiting casts, focal injections and by surgery. Treatment of spasticity with oral medicines can be useful only in selected patients where a generalized reduction in body tone is needed. Baclofen, Diazepam, Tizanidine and Dantrolene are currently approved for use in patients with spasticity. Medication plan should be devised seeking a balance between improved function, patient satisfaction and possible side effects [7].
Diazepam and Baclofen are the oldest and most commonly prescribed drugs in cases of spastic CP. Both have Gaba Amino Butyric Acid (GABA) mediated action. Diazepam exhibits its effects by increasing the inhibitory effects of neurotransmitter GABA at CNS synapses. Most commonly reported adverse reactions are drowsiness, muscle weakness and ataxia. Prolonged use can produce physical dependence. American Academy of Neurology (AAN) recommends Diazepam should be considered as a short term antispasticity treatment in children with CP. Baclofen is an agonist that has presynaptic and postsynaptic effects on monosynaptic and polysynaptic pathways by binding to GABA B receptors. The most common adverse reaction is transient drowsiness, others are dizziness and weakness. Baclofen should be tapered gradually at higher doses to prevent withdrawal symptoms like hallucinations, seizures and increased spasticity. AAN recommends: There is insufficient evidence to support or refute the use of oral baclofen for the treatment of spasticity or to improve motor function in children with CP [8].
There are several studies stating efficacy of Diazepam and Baclofen as antispastic agent in CP. However, there are very few studies comparing effectiveness of Diazepam and Baclofen in spastic CP.
Aim
Study was aimed to assess and compare outcome of oral Diazepam and Baclofen in spastic cerebral palsy children in terms of extent of reduction of spasticity and side effects profile.
Materials and Methods
Randomized comparative follow-up study was conducted for a period of one year i.e. May 2010 to May 2011 in either group. Randomization was done by randomly generated number from computer and sealed envelope of treatment was allocated to patient by Chief Supervisor. An informed consent was taken from parents after explaining possible side effects and asked to contact one of research team member if any adverse events occurred. Experimentation was approved by the ethical & institutional review board of VMMC and Safdarjang Hospital, New Delhi and conforms to Helsinki Declaration.
Study Participants
Sixty seven children with diagnosis of spastic CP, who fulfilled following inclusion and exclusion criteria were eligible for the study. Children aged 2-18 years with Modified Ashworth’s Scale [9,10] score of 1+ or higher in atleast one arm and one leg or both legs were included. Children having spasticity due to progressive disorders such as leukodystrophy, already taking oral antispastic drugs, history of Botox injection and orthopedic procedure performed earlier were excluded.
The diagnosis was made after elaborative history, symptoms and clinical examination. To detect a difference in MAS with a 5% significance level and a power of 80%, a sample size of 46 patients per group was necessary. To recruit this number of patients a 12-month inclusion period was anticipated however required number could not be achieved.
Treatment Interventions
Group A was given Diazepam tablet initially 0.1mg/kg/day in divided doses with weekly increment of 0.1mg/kg upto a maximum of 0.8mg/kg/day in opaque bottle.
Group B received Baclofen tablet initially 2.5mg t.i.d in children < 8 years and 5mg t.i.d. in children > 8 years with weekly increment of 5 mg to a maximum of 40 mg / day in former and 60 mg/ day in latter in opaque bottle [11].
Compliance check was done by confirming from guardian and return of empty bottles.
Assessment and Outcome Measures
Baseline clinical and demographic data were recorded for each child including age, weight, spasticity distribution and Gross Motor Functional Classification System (GMFCS) level [12]. Baseline investigations including liver function test and kidney function test were recorded [11]. Following outcome measures were recorded at pretreatment, one month and third month by Principle Investigator:
Spasticity on Modified Ashworth’s Scale (MAS) for the following muscle groups (flexors/extensors): Elbow, wrist, knee and ankle. MAS has poor to good intrarrater reliability and moderate to good interrater reliability [9]. MAS has conflicting results for validity in different studies but is still most practical and cheap scale for clinical use [10].
Range of Motion (Flexion-Extension arc) in elbow, wrist, knee and ankle was assessed with a universal goniometer. Degree was used as standard unit to measure ROM.
Adverse effect profile: Physician allocating group knew drug given but patient, outcome assessor and data analysts were kept blinded to allocation. All assessments (GMFCS, MAS, ROM) were done by Principal Investigator.
Statistical Analysis
Descriptive statistics including mean and Standard Deviation (SD) were found for each quantitative variable. Frequency distributions were found for each of the qualitative variable. For quantitative data, the mean values across various follow-ups were compared using the Non-parametric Wilcoxon signed ranks test. Inter group comparison was done using Mann-Whitney U test. The results were considered significant at 5% level of significance i.e. p < 0.05.
Results
Disposition and Demographics
Out of 67 patients enrolled in the study, 34 were included in Diazepam group and 33 were enrolled in Baclofen group. Four patients (2 in each group) were lost to follow-up and one patient each in Baclofen as well as Diazepam group had to stop the drug therapy due to excessive day time drowsiness. One patient in Diazepam group had to stop drug due to urticaria. Sixty patients completed the study protocol and the following observations were made [Table/Fig-1].
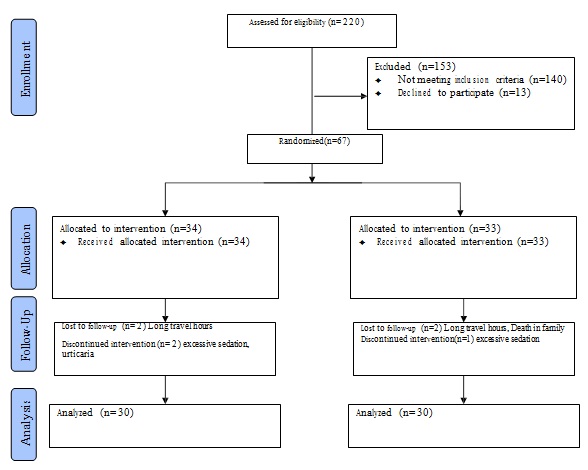
Patients in the study ranged from 2-14 years in both the groups. Each group had 30 patients with mean age of 5 years 7 months ± 2.6 in Diazepam group and 5years 5months ± 2.7 in Baclofen group. Sex ratio was observed to be M:F = 2:1 in both groups. Each group had patients only of middle and lower class with ratio of 1:1 in Diazepam group and 2:1 in Baclofen group as per Kuppuswamy index [13]. Both the groups were comparable on baseline characteristics shown in [Table/Fig-2].
Demographic and clinical characteristics of patients.
| Diazepam group(n=30) | Baclofen group(n= 30) | Total(n=60) |
|---|
| Age (years)Mean (±SD) | 5.7± 2.62 | 5.5 ±2.71 | 5.56 ±2.64 |
| Sex ratio (M:F) | 2:1 | 2:1 | 2:1 |
| Socioeconomic status(Middle: Lower) | 1:1 | 2:1 | 1.4:1 |
| Topographical Distribution |
| Spastic diplegia | 21 | 18 | 39 |
| Spastic quadriplegia | 7 | 11 | 18 |
| Spastic hemiplegia | 2 | 1 | 3 |
| GMFCS Level |
| Level I | 3 | 2 | 5 |
| Level II | 11 | 12 | 23 |
| Level III | 10 | 10 | 20 |
| Level IV | 5 | 4 | 9 |
| Level V | 1 | 2 | 3 |
values are represented as mean ± standard deviation or number
M:F, Male: Female; GMFCS, Gross Motor Functional Classification System
Spastic diplegia comprised the most common group i.e 65%, 30% had quadriplegia and 5% had hemiplegia. In the study population maximum 46.66% patients were observed functioning at level I / II, 33% at level III and 20% at level IV / V of GMFCS criteria. There was no difference between the two groups [Table/Fig-2].
Spasticity Reduction
The mean MAS score for each group at pretreatment and follow- up course is shown in [Table/Fig-3].
Improvement in Mean MAS Score (N =60).
| Drug | Pre- Treatment | 1 month | 3 months | p-value(Intra group) | p-value(Inter group) |
|---|
| 0-1 month | 1-3 month | Pre- Treatment | 0.28 |
|---|
| Diazepam(n= 30) | 1.96±0.4CI : 1.78-2.15 | 1.63±0.40CI : 1.48 -1.79 | 1.41±0.36CI : 1.28-1.55 | 0.0001 | 0.0001 | 1 month | 0.48 |
| Baclofen(n=30) | 1.84±0.64CI : 1.60-2.08 | 1.57±0.59CI : 1.35-1.79 | 1.31±0.48CI : 1.13-1.50 | 0.0001 | 0.0001 | 3 months | 0.22 |
Values are presented as mean ± standard deviation.
MAS, Modified Ashworth Scale.
Intra group : Wilcoxon signed ranks test
Inter group : Mann-Whitney U test
Significant p-value < 0.05%
The baseline MAS score was 1.96±0.4 in Diazepam group and 1.84±0.64 in Baclofen group. At first and third month both groups showed significant (p-value < 0.001) improvement in MAS score compared to baseline (MAS score 1 and 3rd mth: Diazepam 1.63±0.40 and 1.41±0.36; Baclofen 1.57±0.59 and 1.31±0.48). Comparison of mean MAS score in two groups was not statistically significant (p-value > 0.05) both at pretreatment and follow-up at first and third month.
Mean ratio of normal range in each group is shown in [Table/Fig-4]. The baseline ROM was 0.93 ±0.038 in Diazepam group and 0.93 ±0.036 in Baclofen group. At first and third month both groups showed significant (p-value < 0.001) improvement in ROM compared to baseline (ROM at 1 and 3rd mth: Diazepam 0.94±0.033 and 0.95±0.027; Baclofen 0.94±0.034 and 0.95±0.025). On comparison for range of motion at pre-treatment, 1 month and 3 months no statistically significant difference was found between the two drugs.
Improvement in Range of Motion (n=60). (Mean ratio of normal range)
| Drug | Pre-Treatment | 1 month | 3 months | p-value(Intra group) | p-value(Inter group) |
|---|
| 0-1 month | 1-3 month | Pre- Treatment | 0.79 |
|---|
| Diazepam(n= 30) | 0.93±0.038CI: 0.92-0.95 | 0.94± 0.033CI: 0.93-0.96 | 0.95± 0.027CI: 0.95-0.97 | 0.004 | 0.000 | 1 month | 0.81 |
| Baclofen(n= 30) | 0.93± 0.036CI: 0.92-0.95 | 0.94± 0.034CI: 0.93-0.95 | 0.95± 0.025CI: 0.95-0.97 | 0.010 | 0.000 | 3 months | 0.92 |
Values are presented as mean ± standard deviation.
Intra group: Wilcoxon signed ranks test
Inter group: Mann-Whitney U test
Significant p-value < 0.05%
Adverse Effects
Majority of patients i.e. 77% in Diazepam and 80% in Baclofen group [Table/Fig-5] had no adverse effects after three months of therapy. Troublesome drowsiness was the commonest side effect in both groups, being more frequent in patients taking Diazepam. Weakness was next most common complaint in either group. The other striking observation was complaint of increased salivation in patients(3/30) taking Diazepam and paresthesia was observed in one patient taking Baclofen. No significant difference was found between two drugs on comparison of adverse effects.
| Adverse Effects | Diazepam | Baclofen |
|---|
| 1 Month | 3 Months | 1 Month | 3 Months |
|---|
| No Side Effect | 14(46.66%) | 23(76.66%) | 16(53.33%) | 24(80%) |
| Drowsiness | 9(30%) | 3(10%) | 6(20%) | 1(3.33%) |
| Weakness | 3 | 1 | 2 | 2 |
| Urinary Frequency | 3 | 1 | 3 | 1 |
| Headache | 2 | 1 | 2 | 0 |
| Constipation | 2 | 0 | 0 | 1 |
| Drooling | 1 | 3 | 0 | 0 |
| Ataxia | 1 | 0 | 0 | 0 |
| Nausea | 0 | 0 | 2 | 0 |
| Behavioral changes | 0 | 0 | 0 | 1 |
| Paresthesia | 0 | 0 | 1 | 0 |
| Urticaria | 1 | 0 | 1 | 0 |
Values are represented as number (%)
Discussion
Most common form of CP is spastic CP. Child’s learning and emotional development is impaired if he is not able to explore his environment. Effective overall management is needed in pursuit of trying normal development of child. The study was conducted using the two most commonly used oral antispastic drugs i.e. Diazepam and Baclofen in patients with spastic CP. The outcome of oral Diazepam with Baclofen was compared in patients with spastic CP in terms of reduction of spasticity, improvement in range of motion and adverse effect profile.
This study confirmed that both Diazepam and Baclofen are highly effective agents to combat spasticity in CP but failed to demonstrate any significant difference between the two drugs. Statistically significant improvement was seen in the mean MAS score at 1 month and 3 months in both the groups. Similar improvements were found in a study [14] in which Diazepam was compared with placebo in 16 patients of CP. The study reported 75% improvement after 6 weeks of Diazepam which was statistically significant. Another study [15] measured effect of Diazepam and placebo in 180 patients with cerebral palsy. They found significant dose dependent decrease in the muscle tone as graded by Modified Ashworth’s Scale in Diazepam group. A study [16] on Baclofen in 23 patients with spasticity of spinal origin showed significant improvement (p < 0.05) in terms of mean change in MAS as compared to placebo. A controlled trial [17] of Baclofen showed significant improvement in spasticity in a group of 20 cerebral palsy patients. A crossover trial [18] compared the two drugs in 37 adult patients presenting with spasticity of different aetiologies. Significant improvement was documented after 2 weeks of therapy in both Baclofen as well as Diazepam group. Either drug was not found to be superior to other in relieving the spasticity.
Pre-Treatment range of motion at elbow, wrist, knee and ankle joints of both the limbs observed in Diazepam and Baclofen group had no significant difference. Statistically significant improvement was seen in the range of motion at 1 month and 3 months in both the groups but there was no significant difference between the two groups at 1 month and 3 months. Study [14] assessing effect of Diazepam, observed that 12 patients out of 16 showed statistically significant improvement in range of motion. Another study [15] found that treatment with Diazepam in cerebral palsy patients achieved significant reduction in hypertonia, increase in range of motion at ankle and improvement in quality of life. Baclofen study [17] found significant improvement in active range of motion as compared to placebo. A double-blind, randomised cross-over pilot study [19] of oral baclofen versus placebo on fifteen children with spastic or spastic/dystonic quadriplegia scored significantly better on the Goal Attainment Scale with baclofen compared with placebo. Modified Tardieu Scale and Pediatric Evaluation of Disability Inventory showed no significant difference between Baclofen and placebo groups. This study demonstrated that in CP children with spastic quadriplegia oral baclofen helps in improving goal-oriented tasks such as transfers. Double blind cross-over study [20] of Baclofen and Diazepam for reduction of spasticity in a heterogenous group of adult patients found that both drugs produced overall improvement and there was no statistically significant difference between the two groups. Also on long term follow up in Baclofen group found worsening of symptoms and signs in 16 out of 18 patients on discontinuation of drug.
The side effects noted in Diazepam group were drowsiness, drooling, ataxia, constipation, headache, weakness, urticaria and increased urinary frequency. In the study population two patients in Diazepam group had to stop therapy, one due to excessive drowsiness and other because of urticaria. The side effects noted in Baclofen group were drowsiness, behavioral changes, constipation, headache, weakness, nausea, paresthesia and increased urinary frequency. One patient in Baclofen group dropped out due to excessive drowsiness. Majority of patients had no side effects with Diazepam or Baclofen after three months of therapy as we used slow titration of both the drugs. In both the groups drowsiness was most common side effect reported for which one patient dropped out in each group. Similar results were found in previous studies [18–20] which concluded that side effects especially excessive day time drowsiness was more common in Diazepam group as compared to Baclofen group.
Our results were consistent with the previous studies, which had suggested that there seems to be little difference between Baclofen and Diazepam in terms of reduction of spasticity and improvement in terms of range of motion.
Diazepam and Baclofen are effective in spasticity management and response to both drugs can be objectively monitored. Possible side effects associated with their use can be minimized by gradual titration both at time of starting and withdrawal of drugs.
Limitations
The present study had a number of limitations, including a small sample size and heterogeneity within the groups in respect to age and grading of spasticity. Follow-up duration was short i.e. only for 3 months.
Conclusion
The present study showed that oral Diazepam and Baclofen are effective antispastic agents that can be used safely in patients with spastic cerebral palsy. Patients in both the groups showed adaptation to the side effects on gradual titration. Comparison between the two drug groups showed no statistical significant difference in outcome measures. No one drug was found superior to the other. The choice for selection of the medication will depend on the cost and availability.
[1]. Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, A report: The definition and classification of Cerebral Palsy April 2006Dev Med and Child Neurol Suppl 2007 109:8-14. [Google Scholar]
[2]. Kuban KCK, Leviton A, Cerebral palsyN Engl J Med 1994 330:188-95. [Google Scholar]
[3]. Stanley F, Blair E, Alberman E, Cerebral Palsies: Epidemiology and Causal Pathways 2000 LondonMac Keith [Google Scholar]
[4]. Johnston MV, Cerebral palsy. In: Kliegman, Stanton, Geme St., Schor, Behrman, edsNelson Textbook of Pediatrics 2012 PhiladelphiaElsevier Saunders:2061-2065. [Google Scholar]
[5]. Sawyer JR, Cerebral Palsy. In: Canale ST, Beaty JH, editorCampbell–s Operative Orthopedics 2007 11th edPhiladelphiaMosby Elsevier:1333-88. [Google Scholar]
[6]. Thompson AJ, Jarrett L, Lockley L, Marsden J, Stevenson VL, Clinical management of spasticityJ Neurol Neurosurg Psychiatry 2005 76:459-63. [Google Scholar]
[7]. Goldstein EM, Spasticity management: An overviewJ Child Neurol 2001 16:16-23. [Google Scholar]
[8]. Delgado MR, Hirtz D, Aisen S, Practice parameter: Pharmacological treatment of spasticity in children and adolescents with Cerebral Palsy (An evidence based review)American Academy of Neurology 2010 74:336-43. [Google Scholar]
[9]. Mutlu A, Livanelioglu A, Gunel MK, Reliability of Ashworth and Modified Ashworth scales in children with spastic cerebral palsyBMC Musculoskelet Disord 2008 9:44 [Google Scholar]
[10]. Maria BF, Hadas S. A Review: The Validity and Reliability of the Modified Ashworth Scale as a Measurement Tool for the Evaluation of Spasticity and its Applicability to Children with Cerebral Palsy. 2005. http://kennisbank.hva.nl/document/220125. Accessed 2 May 2015 [Google Scholar]
[11]. Edgar TS, Oral Pharmacotherapy of childhood movement DisorderJ Child Neurol 2003 18:40-49. [Google Scholar]
[12]. Palisano R, Rosenbaum P, Walter S, Development and reliability of a system to classify gross motor function in children with cerebral palsyDev Med Child Neurol 1997 39:214-23. [Google Scholar]
[13]. Mishra D, Singh HP, Kuppuswamy’s socioeconomic status scale—A revisionIndian J Pediatr 2003 70:273-74. [Google Scholar]
[14]. Engle HA, The Effect of Diazepam in children with cerebral palsy: A double blind studyDev Med Child Neurol 1966 8:661-67. [Google Scholar]
[15]. Mathew A, Mathew MC, Macaden AS, Antonisamy B, Ernest KM, Measurement of the angle of plantar flexion An objective way of assessing muscle relaxation in children with spastic cerebral palsyIJPMR 2007 18(1):11-14. [Google Scholar]
[16]. Hudgson P, Weightman D, Baclofen in the treatment of spasticityBrit Med J 1971 4:15-17. [Google Scholar]
[17]. Milla PJ, A Controlled trial of Baclofen in children with Cerebral PalsyJ Int Med Res 1977 5:398-405. [Google Scholar]
[18]. Cartilidge NEF, Hudgson P, Weightman D, A Comparison of Baclofen and Diazepam in the treatment of spasticityJour of the Neurol Sciences 1974 23:17-24. [Google Scholar]
[19]. Scheinberg A, Hall K, Lam LT, Flaherty SO, Oral Baclofen in children with Cerebral Palsy: A double blind cross over pilot studyJ of Paed and Child Health 2006 42:715-20. [Google Scholar]
[20]. Roussan M, Terrence C, Framm G, Baclofen versus diazepam for the treatment of spasticity and long term follow up of Baclofen therapyPharmatherapeutica 1985 4:278-84. [Google Scholar]