Systemic Lupus Erythaematosus (SLE) is a systemic autoimmune disease that may present in vastly different ways. Haematological alterations such as anaemia, neutropenia and thrombocytopenia are frequent in SLE [1]. Anaemia of chronic disease is the most common type of anaemia seen in SLE; attributed to its complex aetiopathogenesis, cytokines, impaired erythropoietin response and auto antibodies against erythropoietin [2].
Nutritional anaemia is a major public health problem in India and is primarily due to iron deficiency as stated by the National Family Health Survey-3 (NFHS-3) data [3]. Hence, pure anaemia of chronic disease in patients presenting with SLE in our country is highly unlikely. For this reason treating these patients for their primary disease does not really relieve them of their haematological complaints; therefore, possibility of additional pathogenesis occurring simultaneously is there.
Routine iron studies include serum ferritin assay which is an acute phase reactant can be falsely elevated in lupus cases. Accordingly, with an aim to identify the type of haematological alterations and also to compare the types of anaemia based on serum ferritin assays and soluble transferrin receptor assays in patients presenting with this autoimmune disorder; we carried out our study. In addition status of Lupus anticoagulant, anti-beta2 glycoprotein1 and C-reactive protein (CRP) levels were checked and their clinical implication along with disease activity score was determined in our study group.
Materials and Methods
A prospective cohort of 30 newly diagnosed ANA positive (immunoflourescence) based on Revised ARA criteria 2007, cases of Systemic Lupus Erythaematosus (SLE) admitted from May 2012 to May 2013 in the Department of Rheumatology were incorporated in the study after taking their consent and ethical clearance was done. Patients with co-morbidities like cardiovascular disease, atherosclerosis, hyper-lipidemia, diabetic gangrene, bleeding disorders and coagulopathies, drug induced auto-immune diseases, syphilis or other sexually transmitted diseases were excluded from the study. Additionally, patients with haematological malignancies or on anticoagulants, iron-therapy, immunosuppressants, non-steroidal anti-inflammatory drugs and those with past history of blood transfusion were also disqualified from study.
Multiple tests were performed on EDTA blood, serum and plasma of these patients. Cut-off values for each parameter were considered as per the standard values [4–6] and their respective kits.
Routine haematological investigations including Hb, CBC (Swelab Alfa Three part haematology analyser), ESR (Westergren’s method), GBP (Leishman’s stain) and Reticulocyte count (Brilliant Cresyl Blue dye) were done on EDTA blood sample. Coagulation studies such as APTT (Siemens Actin), PT (Siemens Thromborel S), Kaolin clotting time (Lupakct Tulip Diagnostics), dRVVT (LA Screen and LA Confirm Tulip Diagnostics), and Mixing studies were performed on citrated samples. Serological tests were done by Semi- auto analyser (Hitachi 911/912) which included LDH (ELITech) and renal function tests (PZ Cormay S.A.). High sensitivity CRP was estimated by Calbiotech hsCRP ELISA. Iron studies: Serum ferritin (Accubind ELISA kit), TIBC, Serum iron, % transferrin saturation, soluble transferrin receptor levels (TSZ ELISA kit) were also performed.
Additional parameters estimated were: anti beta 2 glycoprotein-1 levels (Dr Fenning ELISA kit), direct & Indirect Coomb’s (Eryclone Anti Human globulin Tulip Diagnostics). All the test results were interpreted based on the respective kit standards.
Clinical symptoms were noted, disease activity score (SLEDAI) was estimated. The results were presented in mean (±SD) and percentages. The Chi-square test was used to compare the categorical variables. The Kruskal-Wallis test was used to compare more than two groups and Tukey’s multiple comparison tests was for pair wise comparisons. The Spearman correlation coefficient was used to find the correlation between two continuous variables. The p-value less than 0.05 were considered as significant. All the analyses were carried out by using SPSS 16.0 version.
Results
Prevalence of SLE was predominant in females (F:M-29:1). The mean age of disease onset was 29 years. A total of 21 out of 30 (70%) patients were found to have anaemia, 7 out of 30 (23%) patients had thrombocytopenia, and 5 out of 30 (16.7%) patients had both anaemia and thrombocytopenia. No patient had leucopoenia. However, 30% (9 out 30) patients were found to have leucocytosis. All anaemic patients were further assessed as per flow diagram in [Table/Fig-1]. [Table/Fig-1] also summarises our results of Iron Deficiency Anaemia (IDA), Anaemia of Chronic Disease (ACD) on routine iron studies and special tests which included soluble transferrin receptor assays (Sol tFR) and Soluble transferrin receptor index (Sol tFR index= Sol tFR/ log ferritin) which were done on non-haemolytic cases. Features of haemolysis (LDH>480IU/l, Indirect bilirubin>2mg/dl, corrected reticulocyte count>2.5% and presence of spherocytes on peripheral smear study) were seen in 2 out of 21 anaemia cases; out of which 1 case showed positive Coomb’s test. As per the diagnostic criteria for anaemia (as calculated from soluble transferrin receptor >5mcg/ml) 3 out of 19 (16%)patients were found to have IDA (soluble transferrin receptor<3 mcg/ml), 5 out of 19 (26%) patient was found to have ACD and 11 out of 19 (58%) patients were found to have anaemia due to co-existing IDA and ACD (soluble transferrin receptor>=3 but <5 mcg/ml) amongst the patients of non-haemolytic anaemia (19 out of 30).
Approach to patients of SLE with Anaemia and distribution of cases of Non-Haemolytic Anaemia classified as per iron studies, solTfR and sol TfR Index.
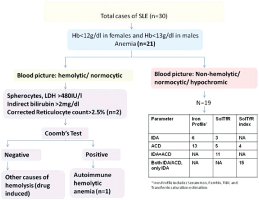
Thus, on sol TFR assays the number of patients with pure ACD reduced from 13 to 5 out of 19(68% to 26%), and, the number of patients with pure IDA reduced from 6 to 3 out of 19 (32% to 16%) while a new group of patients were defined who had co-existing IDA and ACD which were 11 out of 19 (58%) in our study. {Agreement=53%, p=0.09}.
Similarly when calculated from soluble transferrin receptor index>1.5mcg/ml) 15(79%) out of 19 patients were found to have co-existing IDA and ACD or only IDA, while 4 (21%) out of 19 patients were found to have pure anaemia of chronic disease (soluble transferrin receptor index<1.5mcg/ml) amongst the patients of anaemia (19 out of 30).
Thus, on sol TFR index the number of patients with pure ACD reduced from 13 to 4 out of 19 (68% to 21%) and the number of patients with pure IDA or with co-existent IDA & ACD could not be determined separately {Agreement=24%, p=0.11}.
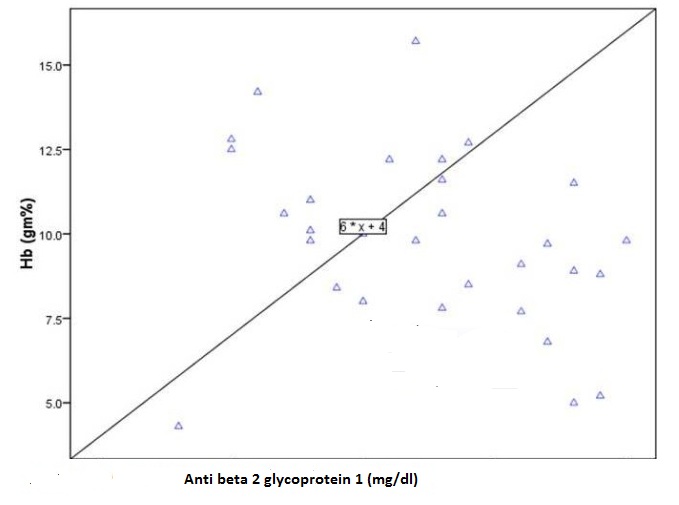
Anti-phospholipid antibody assays were done; three patients showed positivity for both LA as well as anti-beta2 glycoprotein-1 positivity (anti beta 2 glycoprotein 1 >1.4mg/dl) while in seven cases only beta2 glycoprotein-1 levels was positive. Out of the 3 patients who showed positivity for both LA and anti-beta2 glycoprotein-1, two patients had history of thrombosis. The patients with high beta2 glycoprotein-1 levels had low haemoglobin as well [Table/Fig-2]. The clinical features of patients were studied and disease activity was determined based on SLEDAI score. Out of all clinical signs and symptoms of SLE, we found history of fever (p<0.0001) and arthritis (p<0.03), to be in statistically significant association with the disease activity scores based on SLEDAI. Serum Ferritin levels, C-reactive protein (CRP) and Erythrocyte Sedimentation Rates (ESR) were compared with disease activity scores calculated by SLEDAI. Association with disease flare was found to be significant with CRP (p=0.04) in comparison to ESR (p=0.35) and Ferritin (p=0.94).
Comparison of Beta 2 glycoprotein 1 and Hemoglobin.
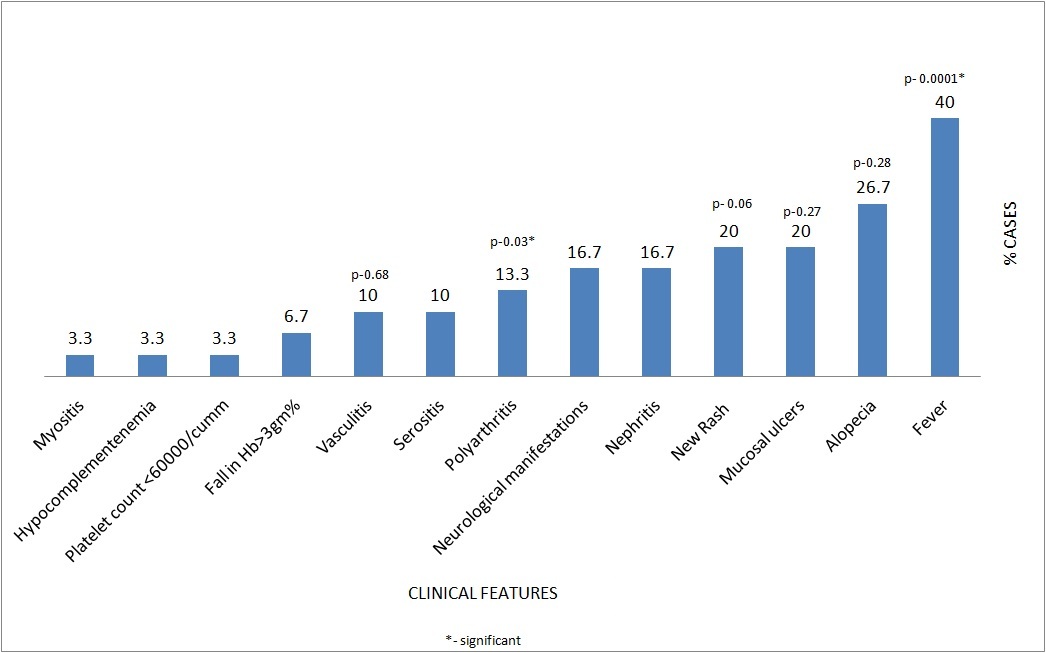
In patients with no flare, fever (91.7%) was the most common presenting symptom followed by history of new rash (41.7%). Patients with mild to moderate flare had polyarthritis, alopecia and mucosal ulcers which were distributed equally (50% each). In patients with severe flare, serositis (21.4%), nephritis (35.7%), neurological manifestations (35.7%) were the most common presenting features [Table/Fig-3].
Distribution of patients.
Discussion
As far as demographic profile of the study group was concerned, female predominance was observed. The increased frequency of SLE among women has been attributed in part to estrogens [7,8]. The mean age of onset of disease of patients in our study was 29 years.
On thorough review of literature; different authors have studied haematological alterations in SLE patients and we found our findings comparable to their results to a great extent [Table/Fig-4] [2,9–14]. The major divergence we did was that we analysed our results for sol TFR as a tool to categorize anaemia in SLE patients that had not been investigated by any of these authors. To the best of our knowledge/information no Indian and western studies on sol TFR levels has been done in SLE patients. However, various investigators like Jain et al., have studied on sol TFR assays and sol TFR index in anaemic patients and compared the results of ferritin from sol TFR assays and sol TFR index [15]. When sol TFR was used to determine the type of anaemia results were more informative when compared to conventional tests used (Serum ferritin levels, total iron binding capacity, % transferrin saturation) and consecutively the newly identified subgroup of patients with IDA and ACD overlap benefited with addition of Iron supplements.
Results of studies performed on haematological alterations on SLE: comparison with our data [2,9–14].
| Parameters | Authors |
|---|
| Malaviyaet al., [9] | Giannouliet al., [2] | SusanneM. et al.,[10] | Voulgareliset al., [11] | Beyan et al.,[12] | Shaikh et al.,[13] | Sasidharanet al., [14] | Presentstudy |
|---|
| Year of study | 1993 | 1996 | 1997 | 2000 | 2007 | 2010 | 2012 | 2013 |
| Prevalence | 3.2 | - | - | | | | - | |
| F:M | 11:1 | - | - | | 85:20 | 27:3 | - | 29:1 |
| Mean age | 24.5y | - | - | 30.1 | | | - | 29y |
| Anaemia | - | 50% | 65% | - | | 93.3% | 62.9% | 70% |
| Thrombocytopenia | - | - | 25% | - | | | 39.8% | 23% |
| Leucopenia | | - | 50% | | | | 15.7% | 0% |
| Mean Hb(gm/dl) | | 10.1 | - | | | | 9.5 | 9.8 |
| ACD | | | | 37.1% | 46% | 40% | | 68% |
| IDA | | | | 35.6% | | 30% | | 32% |
| Autoimmune Haemolytic anaemia | | 30-40% | | 14.4% | | 16.66% | 27.9% | 4.7% |
Ferritin being an acute phase reactant, diagnosis of iron deficiency in hospitalized or ill patients can be missed as; such patients have normal or increased ferritin values even when iron deficient. The low sensitivity of ferritin for iron deficiency in these patients may require an invasive procedure such as bone marrow biopsy and/or a trial of iron therapy to differentiate iron deficiency from other causes of anaemia [16]. To overcome this drawback of ferritin as a marker of decreased marrow Iron stores, many serum markers have been investigated till date. Kohgo et al., proposed that the soluble transferrin receptor, a truncated form of the membrane-associated transferrin receptor as a sensitive indicator of iron deficiency and is not an acute-phase reactant [17]. Since, our study group consisted of SLE patients, where ferritin could be raised as an acute phase reactant, we additionally performed sol TFR assays on their samples to reduce the false positive cases of ACD.
As summarized in [Table/Fig-1] by sol TFR assays the number of patients with pure ACD reduced from 68% to 26% and the number of patients with pure IDA reduced from 32% to 16%; 58% patients with co-existing IDA & ACD were identified {Agreement=53%, p=0.09}. By sol TFR index the number of patients with pure ACD reduced from 68% to 21% and the rest of the patients were categorised as pure IDA or with co-existent IDA & ACD which could not be determined separately {Agreement=24%, p=0.11}.
Based on our data we conclude that the sol tFR levels along with the sol tFR index can be very useful in differentiating pure IDA, ACD and identifying those patients who had co-existing ACD with IDA, thus providing a non-invasive alternative to bone marrow iron.
Usual findings like thrombosis in cases with positive LA (2/3) and significant correlation between fever and polyarthritis with disease severity and flare was noted in our study group. A significant negative correlation between Beta2glycoprotein1 and haemoglobin levels has been reported by Suszek et al., (p = 0.0003; r = -0.67) [18]. We also found similar correlation though insignificant (p=0.12), which can be due to small sample size (n=30).
C reactive protein was found to be raised in 18 out of 30 (60%) patients while 12 out of 30 (40%) patients had normal levels. CRP, Ferritin and ESR levels were compared with the disease activity based on SLEDAI score among which; only CRP levels were found to be in statistically significant association with the disease activity score SLEDAI (p <0.04). This was very close to the findings of Amezcua et al., who concluded that the disease activity score in SLE was positively correlated with CRP (p = 0.19, 0.0007 to 0.36; p = 0.04) [19]. But in contrast to Amezcua et al., who found raised ESR in addition to CRP to be significantly correlating with disease activity score (p = 0.23, 95 % CI 0.04 to 0.4; p = 0.01) we could not get such an association. This variation in results may be due to a small sample size (n=30) in our study and also to the variation of organ involvement with different disease activity scores (by SLEDAI).
Conclusion
Based on our results we recommend sol TFR as a non-invasive diagnostic tool for definitive identification and typing of anaemia and hence a better guide to the immunologists treating such patients. We also reaffirm that CRP but not ESR levels co-relate with disease severity scores of our cases with SLE. Anaemia followed by thrombocytopenia was the most prevalent haematological alteration in our study group. Sol tFR is superior to ferritin as a non-invasive parameter for classifying anaemia in SLE patients. High CRP values correlate with high disease activity scores.