Therefore, this study was carried out in a tertiary care teaching hospital situated in north east India, typical of nearly 250 such teaching hospitals of the country, to compare the burden, aetiology and short term outcome of sepsis treated in ICU and general MW settings.
Materials and Methods
The present study was a hospital-based prospective, analytical observational study, carried out between July 2012 and June 2013, in patients with sepsis admitted to general MW and medical ICU of Guwahati Medical College & Hospital, Guwahati, a tertiary care hospital in northeast India catering to several north eastern states of the country.
Sepsis was diagnosed using ’International Sepsis Definitions Conference’ criteria [16]. During the one year period of the study of the total 17441 admissions in the general MW, 1831 patients were diagnosed to have sepsis, giving a hospital incidence of 10.5% sepsis in the general MW. Of these 1831 patients, 150 patients were selected randomly for the study, having satisfied the selection criteria and agreeing to participate in the study. Similarly, during the same period, of the total 558 admissions to the medical ICU, 108 had sepsis, giving an incidence of 19.3% sepsis in the medical ICU. Of these, 95 patients, who satisfied the selection criteria and agreed to participate, were enrolled in the study.
Inclusion criteria
Any patient > 18 years admitted to the general MW and medical ICU with clinical and laboratory evidence of sepsis as per the International Sepsis Definitions Conference criteria [16].
Exclusion criteria
Postoperative cases of sepsis.
Posttraumatic cases of sepsis.
Patients with sepsis initially admitted to general wards and then subsequently transferred to ICU or vice versa.
Patients with other potentially life threatening disease or condition, like cerebrovascular accident, myocardial infarction, fulminant hepatic failure, etc were excluded from the study.
Detailed history, clinical examination and laboratory tests (complete blood counts, urine analysis, blood urea, serum creatinine, blood glucose, liver function tests, coagulogram), inflammatory markers (ESR/CRP), chest x-ray, ultrasonography abdomen, CT scan of appropriate region (as indicated), along with cultures from appropriate samples (urine, sputum, serous fluids and/or blood were done in all cases to establish the diagnosis of sepsis and its severity [16], viz: (i) sepsis; (ii) severe sepsis; or (iii) septic shock. Antibiotic sensitivity was done on Muller-Hilton agar plates using standard Clinical and Laboratory Standards Institute (CLSI) guidelines. Demographic data, clinical features, relevant laboratory parameters and the clinical course of the patient were recorded in a pre-tested structured proforma.
Ethics
Ethical clearance was taken from the Institutional Ethical Committee and written informed consent was taken from all the patients included in the study.
Statistics
Sample size: The confidence interval was kept at 95%, with allowable error of 5% and taking an incedence rate of 10.5 in the MW the required sample size for the study for MW was calculated to be 144, which was rounded off to 150. Similarly for the ICU, taking an incedence rate of 19.3 the estimated sample size was found to be 239. However, due to constraints of availability of patients in the ICU, only 95 patients could be enrolled in the study.
Statistical Analysis
Statistical Analyses were done using Statistical Package for Social Survey (SPSS) for Windows version 17.0. Chi-square test/Fisher’s-exact test was used for comparing ratios. A ’p-value’ <0.05 was considered as statistically significant. The results were tabulated and graphically represented using Microsoft Office for Windows 2008.
Results
Based on the selection criteria, 245 patients with sepsis, 150 from general MW and 95 from the medical ICU were enrolled in the study. Of the 150 patients from MW, 57.3% were males with a male: female ratio of 1.34:1. Of the 95 ICU patients, 62% were males with a male: female ratio of 1.63:1. Majority of cases in both groups were above 50 years [Table/Fig-1]; the median age was 56.7 years in general MW and 59.2 years in ICU.
Age distribution of patients with sepsis in MW* and ICU†.
| Age (in years) | No of patients with sepsis (%) | p-value |
|---|
| MW (n=150) | ICU (n=95) |
|---|
| 18-30 | 8 (5.3) | 4 (4.2) | 0.71 |
| 31-40 | 15 (10.0) | 6 (6.3) | 0.64 |
| 41-50 | 28 (18.6) | 17(17.9) | 0.88 |
| 51-60 | 35 (23.3) | 19(20.0) | 0.79 |
| 61-70 | 45 (30.0) | 30 (31.6) | 0.94 |
| ≥71 | 19 (12.6) | 19(20.0) | 0.37 |
*Medical wards; †Intensive care unit
Baseline parameters in terms of frequency of different presenting symptoms, co-morbidities and source of sepsis were statistically similar in the two groups of patients [Table/Fig-2]. Fever was the most common presenting symptom (95.3% in MW and 95.8% in ICU), followed by cough, altered sensorium, dyspnoea, urinary symptoms (dysuria, increased frequency, or decreased urine output), and gastrointestinal symptoms (loose stool, vomiting, and pain abdomen). Associated co-morbidities were present in 53.3% of paitents MWs and in 57.8% in ICU. The most commonly associated co-morbidities in both MW and ICU were diabetes mellitus (29.3% and 33.7% respectively) and chronic obstructive pulmonary disease (20.6% and 28.4% respectively), followed by hypertension, chronic kidney disease, chronic liver disease, benign prostatic hypertrophy and malignancy. Respiratory tract infection was the major source of sepsis in both MW (48.6%) and ICU (53.7%), followed by the urinary tract, primary blood stream infections and gastrointestinal tract.
Presenting symptoms, co-morbidities and source of sepsis in patients in MW* and ICU†.
| Parameter | No of patients (%) | p-value |
|---|
| MW (n=150) | ICU (n=95) |
|---|
| Presenting symptoms |
| Fever | 143 (95.3) | 91 (95.8) | 0.86 |
| Urinary symptoms | 42 (28.0) | 25(26.3) | 0.94 |
| Cough | 49 (32.6) | 34 (35.8) | 0.79 |
| Dyspnea | 31 (20.6) | 29 (30.5) | 0.23 |
| Abdominal symptoms | 19 (12.6) | 15 (15.8) | 0.86 |
| Altered sensorium | 35 (26.9) | 38 (40) | 0.09 |
| Co-morbidity | 80 (53.3) | 55 (57.8) | 0.55 |
| Diabetes | 44 (29.3) | 32 (33.7) | 0.57 |
| Chronic Obstructive Pulmonary Disease | 31 (20.6) | 27(28.4) | 0.23 |
| Chronic Kidney Disease | 18 (13.8) | 17 (17.9) | 0.28 |
| Chronic Liver Disease | 13 (8.6) | 10 (10.5) | 0.65 |
| Hypertension | 24 (16.0) | 19(20.0) | 0.49 |
| Malignancy | 10 (6.6) | 8 (8.4) | 0.64 |
| Benign Prostatic Hypertrophy | 21 (14) | 15 (15.8) | 0.79 |
| Source of sepsis |
| Respiratory tract | 73 (48.6) | 51 (53.7) | 0.77 |
| Urinary tract | 30 (20.0) | 17(17.9) | 0.92 |
| Gastrointestinal tract | 11 (7.3) | 6 (6.3) | 0.76 |
| Primary blood stream infection | 21 (14.0) | 11(11.6) | 0.81 |
| Soft tissue infection | 5 (3.3) | 4 (4.2) | 1.20 |
| Others / unspecified | 10 (6.6) | 4 (4.2) | 0.86 |
*Medical wards; †Intensive care unit
Microbiological culture from appropriate specimen (as determined by clinical presentation), e.g. sputum, urine, stool, pus, ascitic or pleural fluid could identify a definite microorganism in 53.3% and 57.9% of cases of sepsis in MW and in ICU respectively. Blood culture was positive in only 27.3% and 22.1% of cases in these two groups respectively. The microorganism isolation rates did not differ significantly between MW and ICU [Table/Fig-3].
Culture positivity rates in sepsis patients in MW* and ICU†.
| Culture | No of patients (%) | p-value |
|---|
| MW (n=150) | ICU (n=95) |
|---|
| Appropriate Specimen Culture | 80 (53.3) | 55 (57.9) | 0.62 |
| Blood Culture | 41(27.3) | 21(22.1) | 0.57 |
*Medical wards; †Intensive care unit
The pattern of isolates from appropriate specimen culture showed a predominance of gram negative organisms both in MW and ICUs. In MW, the most common organism responsible for sepsis was Klebsiella spp. (30.0%), followed by Staphylococcus aureus (20.0%), Pseudomonas aeruginosa (18.7%) and Escherichia coli (16.2%), while in ICU patients, Pseudomonas aeruginosa (32.7%) was the predominant organism followed by Klebsiella spp (22%), Staphylococcus aureus (18.1%) and Escherichia coli (14.5%), [Table/Fig-4]. In cases with positive blood culture the pattern of isolation of pathogens was similar both in MWs and ICU. Pseudomonas aeruginosa (29.2% in MW and 28.6% in ICU) was the most common organism isolated from blood culture followed by Klebsiella spp, Staphylococcus aureus and Escherichia coli [Table/Fig-5].
Isolates from microbiological culture from appropriate specimen in sepsis patients in MW* and ICU†.
| Type of pathogen | No of patients with positive culture of appropriate specimen (%) | p-value |
|---|
| MW (n= 80) | ICU (n = 55) |
|---|
| Klebsiella spp | 24 (30.0) | 12 (22.0) | 0.38 |
| Pseudomonas aeruginosa | 15 (18.7) | 18 (32.7) | 0.36 |
| Escherichia coli. | 13 (16.2) | 8 (14.5) | 0.80 |
| Staphyloccus aureus | 16 (20.0) | 10 (18.1) | 0.80 |
| Enterococcus spp. | 7 (8.7) | 5 (9.1) | 0.65 |
| Others | 3 (3.7) | 2 (3.6) | 0.80 |
*Medical wards; †Intensive care unit
Microbial isolates from blood cultures in sepsis patients in MW* and ICU†.
| Type of pathogen | No of patients with positive blood culture (%) | p-value |
|---|
| MW (n = 41) | ICU (n = 21) |
|---|
| Pseudomonas aeruginosa | 12 (29.2) | 6 (28.6) | 0.80 |
| Klebsiella spp | 9 (21.9) | 4 (19.0) | 0.80 |
| Escherichia coli. | 7 (17.0) | 4 (19.0) | 0.79 |
| Staphyloccus aureus | 8 (19.5) | 4 (19.0) | 0.81 |
| Enterococcus spp. | 3 (7.3) | 2 (9.5) | 0.78 |
| Others | 2 (4.8) | 1 (4.9) | 0.43 |
*Medical wards; †Intensive care unit
In the MW, cephalosporins (67.3%) were the highest used antibiotics followed by quinolones (51.5%), piperacillin-tazobactum (43.3%) and linezolid (42.6%). In ICU, carbapenems (62.1%) were found to be the predominantly used antibiotics, followed by linezolid (55.8%), quinolones (42.1%) and cephalosporins (40.0%). The use of carbapenems and linezolid was significantly higher in ICU (p<0.01 & p=0.04 respectively) whereas the use of cephalosporins was significantly higher in General wards (p<0.01). There was no significant difference in use of other antibiotics between MW and ICU [Table/Fig-6]. A significantly higher number of antibiotics were used in ICU compared to MW. While most of the patients in MW received two antibiotics (69.3%), a majority of those in ICU received three antibiotics (55.8%), the difference being statistically significant. Use of four antibiotics was also significantly higher in ICU (17.9%) (p=0.02) [Table/Fig-7].
Pattern of antibiotic usage in sepsis patients in MW* and ICU†.
| Type of antibiotic | No of patients (%) | p-value |
|---|
| MW (n=150) | ICU (n=95) |
|---|
| Carbapenem | 22 (14.6) | 59 (62.1) | <0.01 |
| Cephalosporins | 101 (67.3) | 38(40) | <0.01 |
| Imidazoles | 52 (34.6) | 34(35.8) | 0.74 |
| Linezolid | 64 (42.6) | 53 (55.8) | 0.04 |
| Piperacillin-Tazobactum | 65(43.3) | 30 (31.6) | 0.06 |
| Quinolone | 77 (51.5) | 40 (42.1) | 0.26 |
| Others | 14 (9.3) | 13(13.7) | 0.36 |
*Medical wards; †Intensive care unit
Number of antibiotics used concurrently in sepsis patients in MW* and ICU†.
| No of antibiotics administered concurrently | No of patients (%) | p-value |
|---|
| MW (n=150) | ICU (n=95) |
|---|
| One | 12 (8.0) | 4 (4.2) | 0.24 |
| Two | 104 (69.3) | 21 (22.1) | <0.01 |
| Three | 24 (16.1) | 53 (55.8) | <0.01 |
| Four | 10 (6.6) | 17(17.9) | 0.02 |
*Medical wards; †Intensive care unit
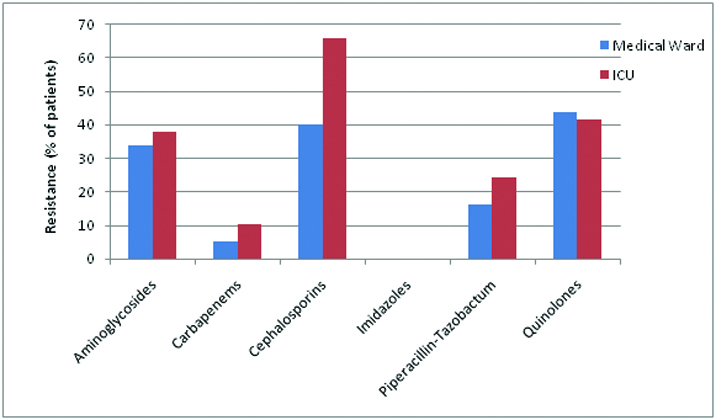
Antibiotic resistance in both ICU and MW was similar (p>0.05). In the MW, maximum resistance was noted to quinolones (43.7%) followed by cephalosporins (40.1%) and aminoglycosides (33.7%) whereas in ICU, resistance was highest to cephalosporins (65.5%), followed by quinolones (41.3%) and aminoglycosides (37.9%). Antibiotic resistance was higher in ICU than in MW for cephalosporins, piperacillin-tazobactum, carbapenems and aminoglycosides, this difference being statistically significant (p<0.01) only for cephalosporins [Table/Fig-8].
Prevalence of antibiotic resistance in sepsis patients in MW and ICU.
While organ dysfunction was a common feature of sepsis in both MW and ICU (65.4% and 77.9% respectively), multi-organ (≥2) dysfunction was present in a significantly higher proportion of patients in ICU as compared to MW (p=0.04) [Table/Fig-9]. The spectrum of the various sepsis syndromes was also different with respect to MW and ICU [Table/Fig-9]. While sepsis and severe sepsis were significantly higher in the MW in comparison to ICU (34.6% vs. 22.1 %, p = 0.03 & 47.3% vs. 26.3%, p<0.01), septic shock was significantly higher in ICU in comparison to MW (51.6% vs. 18.0%, p<0.01).
Frequency of number of organ dysfunction and various sepsis syndromes in MW* and ICU†.
| No of patients (%) | p-value |
|---|
| MW (n=150) | ICU (n=95) |
|---|
| No of Organ Dysfunction |
| None | 52 (34.6) | 21 (22.1) | 0.03 |
| One | 40 (26.7) | 21 (22.1) | 0.57 |
| Multiple (≥2) | 58 (38.7) | 53 (55.8) | 0.04 |
| Sepsis syndrome |
| Sepsis | 52 (34.6) | 21 (22.1) | 0.03 |
| Severe sepsis | 71 (47.3) | 25 (26.3) | <0.01 |
| Septic shock | 27 (18.0) | 49(51.6) | <0.01 |
*Medical wards; †Intensive care unit
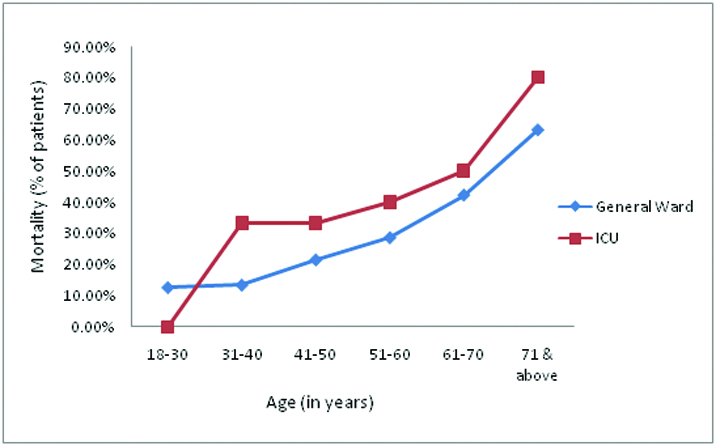
The overall mortality was significantly higher in ICU than MW (48.4% vs. 32.6%, p=0.041). In both the groups the age specific mortality showed a sharp rise, which was proportional to the age of the patients [Table/Fig-10]. Most of the deaths occurred in patients aged above 60 years, both in MW (63.2%) and ICU (66.6%). Above the age of 70 years, the in-hospital mortality in sepsis rose to 63.1% and 80% in MW and ICU respectively.
Age-specific mortality in patients with sepsis in MW and ICU.
There was a progressive rise in the mortality with increasing severity of the sepsis in both MW and ICU without any statistically significant difference between the two groups [Table/Fig-11]. In both MW and ICU, sepsis (without hypotension or organ dysfunction) was associated with the least mortality (11.5% and 19.0%), followed by severe sepsis (39.4% and 48%), and septic shock (55.5% and 61.2%). Likewise, there was a higher mortality with increasing number of organ dysfunction in both MW and ICU without any statistically significant difference between the two groups [Table/Fig-11]. Sepsis with multi organ (≥2) dysfunction had the highest mortality in both MW and ICU (55.1% and 64.2% respectively)
Mortality in relation to severity of sepsis and organ dysfunction in patients in MW* and ICU†.
| Parameter | MW | ICU | p-value |
|---|
| Total no of patients (n=150) | Mortality (%) | Total no of patients (n=95) | Mortality (%) |
|---|
| Severity of Sepsis |
| Sepsis | 52 | 6 (11.5) | 21 | 4 (19.0) | 0.39 |
| Severe Sepsis | 71 | 28 (39.4) | 25 | 12 (48.0) | 0.45 |
| Septic Shock | 27 | 15 (55.5) | 49 | 30 (61.2) | 0.63 |
| No of Organ Dysfunction |
| None | 52 | 6 (11.5) | 21 | 4 (19.0) | 0.38 |
| One | 40 | 11 (27.5) | 21 | 8 (38.1) | 0.39 |
| Multiple (≥2) | 58 | 32 (55.1) | 53 | 34 (64.2) | 0.40 |
*Medical wards; †Intensive care unit
The average duration of hospital stay in sepsis as a whole was significantly shorter among the survivors in MW as compared to ICU (7.3 versus 11.0 days, p<0.01). On sub-grouping the patients into different sepsis syndromes, the duration of hospital stay in MW was shorter than ICU in all the three categories, with these differences being statistically significant in severe sepsis (p=0.048) and septic shock (p=0.041) [Table/Fig-12].
Average duration of hospital stay in different sepsis syndromes in MW* and ICU†.
| Sepsis syndrome | No of patients discharged alive (%) | Average duration of hospital stay‡ (in days) | p-value |
|---|
| MW (n=101) | ICU (n=49) | MW | ICU |
|---|
| Sepsis | 46 (45.5) | 17 (34.7) | 4.9 | 6.2 | 0.092 |
| Severe sepsis | 43 (42.6) | 13 (26.5) | 6.3 | 9.1 | 0.048 |
| Septic shock | 12 (11.9) | 19 (38.8) | 10.7 | 17.7 | 0.041 |
| Overall sepsis | 101 (100) | 49 (100) | 7.3 | 11.0 | <0.01 |
*Medical wards; †Intensive care unit; ‡ in patients discharged alive
Discussion
The present study was conducted with the objective of analysing the spectrum of sepsis in patients in general MW and ICU and to compare the burden, aetiology and short term outcome of sepsis treated in these two settings. Sepsis is gradually becoming a disease of the elderly in which age, load of micro-organisms and their virulence may specifically affect the pathophysiology of the disease. In the present study too, sepsis was found to be significantly higher in older patients as compared to younger patients both in MW and ICU. A trend towards increasing age of septic patients has been documented, with more than 60% of the patients with severe sepsis being aged above 65 years [17]. In the present study, it was seen that sepsis was more common in men, with a male to female ratio of 1.34: 1 and 1.63: 1 in the MW and ICU respectively. An earlier study from India also showed that sepsis is more common in males [5].
The frequency of different presenting symptoms, co-morbidities and source of sepsis were statistically similar in the two groups of patients. Fever was found to be the predominant symptom in both settings (95.3% in MW and 95.8% in ICU), followed by cough, and altered sensorium, urinary symptoms, dyspnoea and gastrointestinal symptoms. A large number of the patients had associated co-morbidities at the time of diagnosis of sepsis in both MW and ICU the commonest being diabetes (29.3% in MW and 33.7% in ICU) and followed by COPD, hypertension and chronic kidney disease. Though similar observation has been made previously by Martin GS et al., who showed that the most frequent co-morbidities were diabetes, hypertension, cancer, and congestive heart failure no significant statistical difference could be established between the associated co-morbidities in the two settings in our study [1]. Respiratory tract infection was the major source of sepsis in both MW (48.6%) and ICU (53.7%), followed by the urinary tract, primary blood stream infections and gastrointestinal tract. A prior study has however shown a pattern of difference between the source of infection between the two settings where patients in non-ICU settings were more prone to have a genito-urinary or soft-tissue infection whereas those in the ICU were more prone to have a respiratory source of infection [18]. A possible cause in this apparent disparity may be due to the fact that our study dealt with patients with medical causes, where surgical causes like soft tissue infections were excluded.
Blood culture was positive in only 27.3% and 22.1% of cases of sepsis in MW and ICU respectively. In clinical practice, at least 50 % of sepsis do not have a microbiologically confirmed of infection [19]. Definitive confirmation is particularly challenging especially for the respiratory tract, which is the most frequent site of infection [6]. The lack of a definite microbiological diagnosis raises uncertainty about the nature of the acute process, thus hampering its prompt therapeutic approach.
Gram negative organisms were predominant isolates in both settings. Prior studies on sepsis from general MW have shown gram negative organisms to be the commonest organism involved [12]. This is in sharp contrast to other studies from ICUs, where gram positive organisms are the predominant organisms, possibly due to the greater use of invasive intravascular catheters and access devices [20]. Cephalosporins (67.3%) and carbapenems (62.1%) were the most commonly used antibiotics in MW and ICU respectively. Use of carbapenems and linezolid was significantly higher in ICU (p<0.01 & p=0.04 respectively) whereas the use of cephalosporins was significantly higher in general MW (p<0.01).
Antibiotic resistance was common both in MW and ICU. In the MW, maximum resistance was noted to quinolones (43.7%) whereas in ICU resistance was highest to cephalosporins (65.5%). A statistically significant difference in the resistance patterns between the two groups was present only for cephalosporins (p<0.01). A significantly higher number of antibiotics were used in ICU compared to MW. While most of the patients in MW received two antibiotics (69.3%), a majority of those in ICU received three antibiotics (55.8%), the difference being statistically significant. Use of four antibiotics was also significantly higher in ICU (p=0.02). The greater degree of resistance to drugs acting on gram negative organisms like cephalosporins is perhaps explained by the empirical use of such antibiotics, which are being supplied free of cost as per hospital policy. In the management of sepsis the timing as well as the choice of antibiotic alters the course of disease progression drastically. However, in resource limited settings with lack of automated culture systems and low yield of traditional culture methods, empirical therapy with one or more antibiotics becomes a necessity.
Multi-organ dysfunction was significantly higher in ICU compared to MW (p=0.04). Sepsis and severe sepsis were significantly higher in MW (p=0.03 & p<0.01 respectively) whereas septic shock was significantly higher in ICU (p<0.01). Prior studies have shown a significant difference in the severity of sepsis in patients admitted to the two different settings with patients in ICU having more severe forms of sepsis in the form of septic shock [18].
There was a sharp rise in mortality with advancing age in both settings, being higher in patients with more severe forms of sepsis and associated multi-organ dysfunction. Further, mortality was significantly higher in ICU as compared to MW (48.4% versus 32.6%, p=0.041). Our results are in agreement with studies from various parts of the world which have reported mortality due to sepsis in the range of 50-60% in ICU settings [20,21]. However, there is very limited published data on sepsis managed in MW. Earlier studies have shown that mortality in sepsis increased with associated organ failure to the range of 70% in patients with multi-organ failure as compared to only 15% without organ failure. It has also been shown previously that mortality in severe sepsis is as high as 59.2% [5].
The overall average duration of hospital stay was significantly shorter among the survivors in MW compared to ICU (7.3 vs. 11.0 days, p<0.01). With respect to the different sepsis syndromes, the average duration of hospital stay was significantly shorter for patients with severe sepsis and septic shock treated in MW as compared to ICU. Though there is a paucity of published literature about the mean duration of hospital stay in sepsis in MW, earlier studies on sepsis in ICU [22] have reported 12 days of hospital stay, which is similar to that of our study.
Limitation
Being a hospital based study our study does not reflect the actual burden of sepsis in the community. Furthermore, the sample size was smaller due to constraints of time and resources. These are the limitations of the study.
Conclusion
Our study was aimed to identify the determinants and outcome of sepsis in general MW and compare with ICU settings. In both settings sepsis was more common with advancing age. However, there was difference in antibiotic usage in the two settings: concurrent use of three or more antibiotics and use of carbapenems & linezolid were significantly higher in ICU as compared to MW. Sepsis treated in MW had significantly lower incidence of multi-organ failure, lower mortality, and shorter duration of hospital stay among survivors, as compared to sepsis treated in ICU.
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit
*Medical wards; †Intensive care unit; ‡ in patients discharged alive