Cardiac Pseudoaneurysm- A Death Defying Entity
Manoj Mathur1, Saryu Gupta2
1 Associate Professor, Department of Radiodiagnosis, Government Medical College, Patiala, Punjab, India.
2 Assistant Professor, Department of Radiodiagnosis, Government Medical College, Patiala, Punjab, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Manoj Mathur, 250, Phulkian Enclave, Patiala-147001, Punjab, India.
E-mail: manojnidhi66@gmail.com
A pseudoaneurysm refers to a contained rupture of the myocardium with a tenuous pericardium walling off the leak. It needs to be differentiated from a true aneurysm by the fact that there is lack of myocardial tissue in the wall of a pseudoaneurysm. The differentiation between the two is pertinent as true aneurysms can be treated medically while pseudoaneurysms require urgent surgical treatment. Untreated pseudoaneurysms carry a high risk of rupture and mortality.
We report a case of cardiac pseudoaneurysm developing in a 46-year-old male who had suffered myocardial infarction four months back. The patient now presented with chest pain and dyspnoea. CECT chest revealed a partially thrombosed large pseudoaneurysm arising from the posterior wall of left ventricle. While the clinical diagnosis of this entity is difficult, CECT plays a pivotal role in the non-invasive detection of pseudoaneurysms.
Free wall rupture, Myocardial infarction, True aneurysm
Case Report
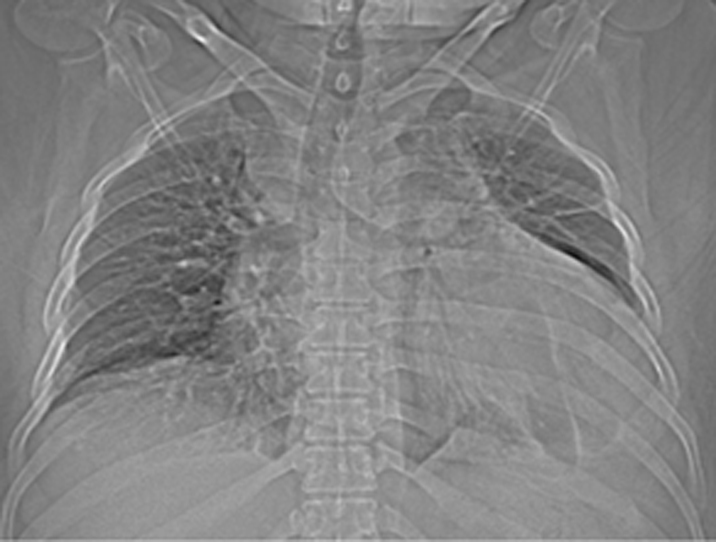
A 46-year-old male patient presented with complaints of chest pain and dyspnoea. Patient had a history of hypertension and had suffered a posterior wall myocardial infarct four months back. Physical examination revealed signs of heart failure. Due to poor window echocardiography could not be undertaken. Scanography revealed cardiomegaly & fluid in left CP angle [Table/Fig-1].
Scanogram shows cardiomegaly with the cardiac silhouette reaching the lateral thoracic wall. Fluid is visualized in the left CP angle.
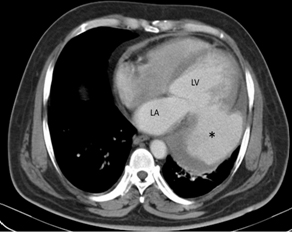
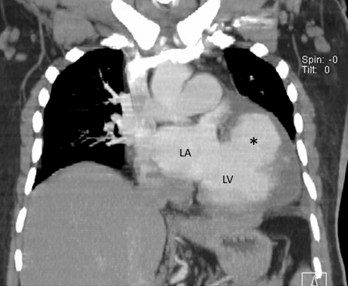
CT scan of the thorax revealed a large pseudoaneurysm [Table/Fig-2] attached to the posterior wall of left ventricle by a narrow neck. The pseudoaneurysm was opacified by contrast and partial thrombosis was seen at the periphery. Left pleural effusion was also noted [Table/Fig-3]. The patient was a case of medical emergency and as soon as the diagnosis was established he was referred to cardiac surgery unit, but unfortunately the patient died during surgery.
CECT shows a large pseudoaneurysm [*] connected to the left posterior free wall of LV by a narrow neck. Blood collection is being walled off by pericardium. Note the thrombosis at the periphery. LA: left atrium. LV: left ventricle.
Coronal CECT image shows the pseudoaneurysm [*] arising from LV. LA: left atrium, LV: left ventricle.
Discussion
A pseudoaneurysm refers to a contained rupture of the myocardium with a tenuous pericardium and fibrous tissue containing the leak. Tear of the myocardial wall is seen in 4% of patients who suffer myocardial infarction [1]. However, pseudoaneurysm develops in only 0.2% of infarcted patients [2]. Pseudoaneurysms compromise the ventricular stroke volume and can be the source of emboli to vital organs like brain and lungs [3].
The most frequent cause of cardiac pseudoaneurysm is transmural myocardial infarction [4]. Transmural infarcts cause pericarditis and pericardial thickening due to fibrosis which is able to contain the leak. Sometimes even the parietal pericardium is breached and the pleura walls off the pseudoaneurysm [5]. Other causes include cardiac surgery, infection or trauma. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa is common after infection or surgery [4].
Most cases present with chest pain, dyspnoea or congestive heart failure. Approximately 10% patients are asymptomatic. Clinical examination may reveal a mitral regurgitation- like murmur due to swirling motion of the blood in the pseudoaneurysm. ECG findings are non-specific [6]. X-ray chest is inconclusive as cardiomegaly is the sole finding. However detection of a para cardiac mass in a post myocardial infarct patient should alert us about the probability of a pseudoaneurysm. Diagnosis is possible by echocardiography but it is a highly operator dependent modality. Although echocardiography is the first investigation performed in patients with cardiac murmur but it is sometimes limited by a poor echo window. Transesophageal echocardiography allows a much better visualization of the cardiac anatomy but has limited availability. CECT is the modality of choice as it offers a quick and easily available option for definitive diagnosis of this entity [7]. Catheter angiography is an invasive technique and runs the risk of causing rupture of the pseudoaneurysm or dislodging the thrombus.
The location is aetiology dependent with the posterior wall being most commonly involved in the post myocardial infarction cases and the anterior wall in post-traumatic cases [8]. The operative site is the most easily compromised location in post-cardiac surgery subset of patients.
Pseudoaneurysms need to be differentiated from true aneurysms which are focal dilatation of the left ventricle due to weakened myocardial wall. The hallmark of true aneurysms is that they are bounded by all the three anatomic layers i.e. endocardium, myocardium and pericardium. On the other hand, a pseudo-aneurysm is bounded only by pericardium. MRI helps to define these three layers and also delineates the infarcted endocardium and myocardium. Delayed intense pericardial enhancement has been identified as an important sign in pseudoaneurysms [9]. This sign has a sensitivity of 100% and a specificity of 83% [9] and is due to pericardial inflammation evoked by blood.
True aneurysms can be managed by conservative medical treatment while the definitive treatment of cardiac pseudoaneurysm is by surgery [10]. Treatment is imperative as untreated cases carry a high mortality of nearly 50% [11]. However Moreno et al., have found that medical treatment carries a low risk of rupture and they advocate surgery only in young patients who have good ejection fraction and no co morbid conditions. They advocate the initiation of anticoagulant treatment in medically treated patients to prevent embolic strokes [12].
Conclusion
Contrast enhanced CT is the modality of choice for diagnosis of a pseudoaneurysm as it allows a quick and definitive diagnosis. Most post myocardial infarct aneurysms are in the posterior wall of left ventricle and are therefore difficult to visualize on conventional echocardiography.
Patients who develop cardiac pseudoaneurysm after cardiac rupture are fortunate to have received a fresh lease of life. Early diagnosis and prompt treatment determine how far this lease would extend. If the patient is young, has no co-morbid conditions and has preserved cardiac function surgery is curative. However medical treatment in the form of anti-hypertensives and drugs to alleviate heart failure can also prolong the life of patient.
[1]. Pollak H, Nobis H, Mlczoch J, Frequency of left ventricular free wall rupture complicating acute myocardial infarction since the advent of thrombolysisAm J Cardiol 1994 74:184-86. [Google Scholar]
[2]. Ando S, Kadokami T, Momii H, Hironaga K, Kawamura N, Fukuiama T, Left ventricular false- pseudo and pseudo aneurysm: serial observations by cardiac magnetic resonance imagingIntern Med 2007 46(4):181-85. [Google Scholar]
[3]. Tran T, Ross BD, Colletti P, Ching RE, Gd-enhanced cardiovascular MR imaging to identify left ventricular pseudoaneurysmJ Cardiovasc Magn Reson 2005 7:717-21. [Google Scholar]
[4]. Sahan E, Gul M, Sahan S, Sokemen E, Guray YA, Tufekcioglu O, Pseudoaneurysm of the mitral-aortic intervalvular fibrosaHerz 2015 40:182-89. [Google Scholar]
[5]. Si D, Shi K, Gao D, Yang P, Ruptured left ventricular pseudoaneurysm in the mediastinum following acute myocardial infarctionEuropean Journal of Medical Research 2013 18:1-4. [Google Scholar]
[6]. Sakai K, Nakamura K, Ishizuka N, Nakagawa M, Hosoda S, Echocardiographic findings and clinical features of left ventricular pseudoaneurysm after mitral valve replacementAm Heart J 1992 124:975-82. [Google Scholar]
[7]. Bozlar U, Yurtsever I, Ugurel MS, Ors F, Nural MS, Tasar M, Multidetector computed tomography in post-traumatic ventricular pseudoaneurysmClin Cardiol 2009 32:72-74. [Google Scholar]
[8]. Yaymaci B, Bozbuga N, Balkanay M, Unruptured left ventricular pseudoaneurysmInt J Cardiol 2001 77:99-101. [Google Scholar]
[9]. Konen E, Merchant N, Gutierrez C, Provost Y, Mickleborough L, Paul NS, True versus false left ventricular aneurysm: differentiation with MR imaging–initial experienceRadiology 2005 236:65-70. [Google Scholar]
[10]. Inoue T, Hashimoto K, Sakamoto Y, Nagahori R, Yoshitake M, Matsumora Y, Spontaneous closure of a large left ventricular pseudoaneurysm after mitral valve replacementGen Thorac Cardiovasc Surg 2014 :1-3. [Google Scholar]
[11]. Garg P, Rajashekar P, Airan B, Giant left ventricular pseudoaneurysm following inferior wall myocardial infarction-A case reportIndian Journal of Thoracic and Cardiovascular Surgery 2013 29:131-33. [Google Scholar]
[12]. Moreno R, Gordillo E, Zamorano J, Almeria C, Garcia-Rubira JC, Fernandez-Ortiz A, Long term outcome of patients with post infarction left ventricular pseudoaneurysmHeart 2003 89(10):1144-46. [Google Scholar]