Solid Pseudopapillary Tumour (SPPT) is a distinctive tumour of low malignant potential with a striking and unexplained predilection for adolescent girls and young women. Hence it is important to distinguish this rare tumour from other pancreatic tumours with similar cytomorphologic features because an accurate preoperative diagnosis is highly desirable since these patients can have long survival with adequate surgery.
We report a case of the rare SPPT of the pancreas in a young girl who presented with nonspecific pain in the abdomen. Radiological investigations revealed a solid cystic mass in relation to the uncinate process of pancreas and third part of duodenum. The mass was diagnosed to be a solid pseudopapillary neoplasm of pancreas on ultrasound guided FNAC. Surgical removal of the pancreatic tumour and detailed histologic study confirmed the cytologic diagnosis.
We present this case because, to date, there are few case reports on the cytological diagnosis of this tumour, about 60 cases, diagnosed by Fine-Needle Aspiration Cytology (FNAC) are reported in the literature. With widespread availability of high-quality imaging systems and a better understanding of its pathology, the number of cases reported in the literature has been steadily increasing in recent years.
In our case, the cytological diagnosis was done even before the detailed imaging findings were available, the cytological features of this tumour are highly characteristic and it is possible to differentiate it from other pancreatic tumours with relative ease.
Case Report
A 17-year-old girl presented with 3-4 months history of upper abdominal pain more on right side. She also had history of postprandial pain, fullness and on and off fever and generalised weakness. Physical examination revealed right hypochondriac tenderness at palpation. Detailed general and systemic examination revealed no other significant findings. The laboratory test including complete blood count, AST, ALT, alkaline phosphatase, bilirubin, random blood sugar and amylase were in normal range.
She underwent ultrasound of the abdomen, which revealed a well defined, heterogenous iso-hypoechoic mass in the area of uncinate process of the pancreas and near the 3rd part of duodenum measuring 6cm×5cmx6cm with multiple tiny cysts. A diagnosis of cystic pancreatic neoplasm was considered.
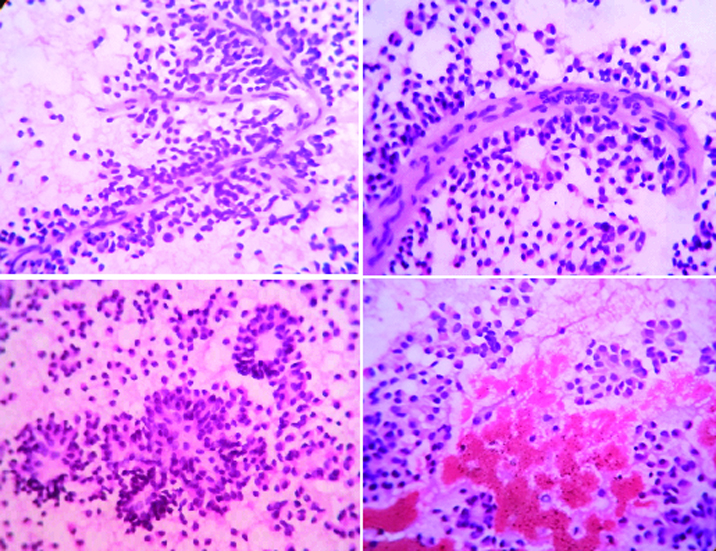
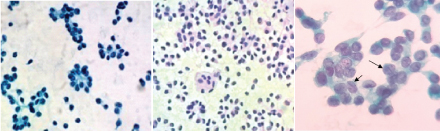
Ultrasound guided fine needle aspiration was performed, smears were prepared and stained using haematoxylin and eosin, Papanicoloau (PAP) and May-Grünwald-Giemsa (MGG) method for cytological evaluation. Hypercellular smears showed numerous delicate branching papillary fronds composed of two or several layers of tumour cells surrounding a thin, fibrovascular core. [Table/Fig-1a,b]. These tumour cells were dispersed singly, also arranged in small scattered clusters and pseudorosettes [Table/Fig-1c&2a]. The tumour cells were uniformly small with scant cytoplasm containing slightly eccentric nuclei [Table/Fig-1c,d&2b] Nuclei were round to oval with small but fairly distinct nucleoli. The nuclear membrane was thin and almost smooth except for occasional infoldings giving a “coffee-bean” appearance. Chromatin was finely granular and evenly distributed [Table/Fig-2c]. Nuclear pleomorphism, indentation, membrane irregularity, mitosis were not observed [Table/Fig-2a,b]. The background was haemorrhagic showed granular debris and foamy histiocytes [Table/Fig-1d]. A cytological diagnosis of solid pseudopapillary neoplasm of the pancreas was made.
Branching papillary fronds with fibrovascular core, rosettes, acini, haemorrhage (H&E ×400).
Round to oval nuclei with fine granular chromatin and nuclear grooves (arrow) (Pap, ×400).
The patient underwent resection of the mass in the pancreatic head and pylorus preserving pancreaticoduodenectomy.
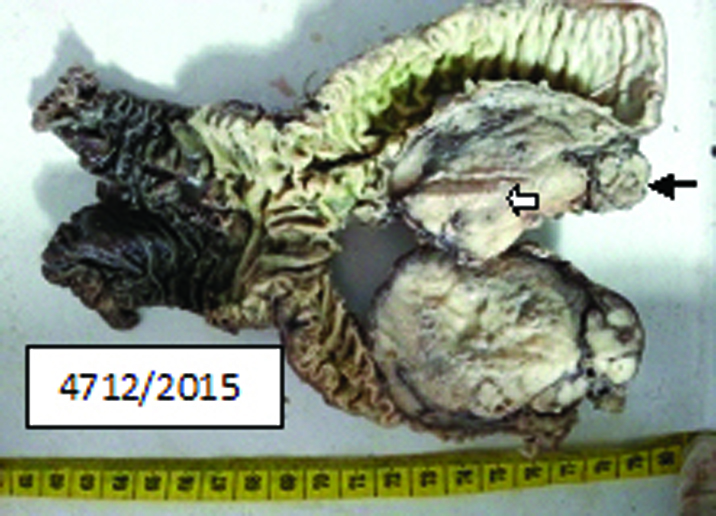
The resected specimen consisted of the head of the pancreas along with tumour and the 3rd part of duodenum. Grossly head of pancreas showed well circumscribed solid cystic greyish white tumour mass closely abutting the serosal surface of the 3rd part of duodenum and a narrow strip of normal pancreatic tissue at one end [Table/Fig-3].
Gross appearance of tumour mass, 3rd part of duodenum. Black arrow denotes normal strip of pancreatic tissue. White arrow denotes tumour mass.
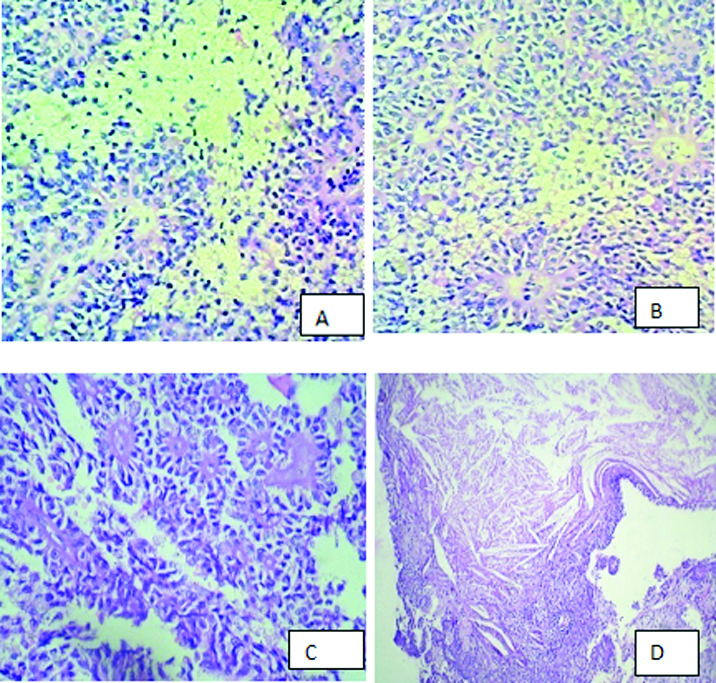
The histological features were mirrored exactly by the cytologic findings. Multiple sections taken from the tumour showed an organoid, microcystic and trabecular pattern with partly solid and partly pseudopapillary structures [Table/Fig-4a-c]. Tumour cells were monomorphic small size with ovoid nuclei, fine chromatin, some with nuclear grooving and clear to eosinophilic cytoplasm. The pseudopapillae, which appeared to be degenerative change, lined by stratified tumour cells covering fibrovascular stalks [Table/Fig-4a]. The perivascular connective tissue and the pseudopapillary core were often hyalinized, foamy cells, areas of necrosis, haemorrhage, cholesterol clefts were also present [Table/Fig-4c&d]. Tumour cells lacked nuclear pleomorphism, and mitotic activity was insignificant. At the periphery of the tumour, compressed exocrine pancreatic tissue was present. Also the tumour was closely attached to the serosal surface of the duodenum. The cytological diagnosis of solid cystic pseudopapillary tumour of pancreas was thus confirmed on histopathology.
Histopathology show pseudopapillary and trabecular pattern. cyst macrophages, necrosis, cholesterol clefts. (H&E ×400)
Discussion
Solid Pseudopapillary Tumour of the Pancreas (SPPT) is a distinctive and rare tumour accounts for 1%-2% of all pancreatic neoplasms [1]. It was first described by Frantz in 1959 and hence called the Gruber-Frantz tumour [2]. The tumour was known by various names like “solid and cystic tumour”, “solid cystic and papillary epithelial neoplasm”, and “solid and papillary tumour” before the present consensus name was defined by the World Health Organization (WHO) in 1996 as a “Solid Pseudopapillary Tumour” of the Pancreas (SPPT) [3]. SPPT mostly occurs in adolescent girls and young women (mean age 25-35 years) [3,4].
An accurate preoperative diagnosis is highly desirable, since local surgical excision is usually curative and this is possible by USG-guided FNA cytology. Bondeson et al., were the first to correctly diagnose SPPT by preoperative FNAC [5]. The characteristic cytomorphological features of SPPT are the branching papillary fronds with monomorphic tumour cells having a palisaded appearance around a fibrovascular core, lack of pleomorphism and mitoses, round to oval nuclei with fine chromatin distribution and occasional nuclear folding [6]. In our case the diagnosis was confirmed on histopathological study of the excised neoplasm.
SPPT has to be differentiated from pancreatic neuroendocrine tumour being the close differential diagnosis, especially if they show a predominant acinar pattern. In the absence of papillae, cytologic features that permits a diagnosis of neuroendocrine neoplasm are dissociated single cells or loose cellular aggregates of uniform, round to polygonal cells with eccentric, round nuclei, a moderate amount of amphophilic cytoplasm, and rosette formation. The nuclei of islets cell tumours have peculiar, fine chromatin aggregates with a salt and pepper appearance and slightly enlarged nucleoli. Some cells contain 2-3 nuclei or a single large nucleus. A papillary structure with vascular core is not seen in islets cell tumours. However, difficulties may be encountered in distinguishing SPPT from an islets cell tumour even on histology, especially if papillary formations are not seen, in such cases immunohistochemistry is helpful [3]. Additionally, these tumours express neuroendocrine markers such as chromogranin, neuron-specific enolase, and synaptophysin which are usually nonreactive in cases of SPPT.
Immunohistochemically, most SPPTs are immunoreactive for α-1-antitrypsin, α-1-Antichymotrypsin (AACT) and neuron specific enolase, CD56, CD10, vimentin and beta-catenin [7]. Cytokeratin (CK) and synaptophysin (Syn), galectin-3 may show focal immunoreactivity by SPPT cells [1]. SPPT is also positive for melanocytic markers (S-100 protein, human melanoma black 45 (HMB45) and melanoma antigen recognized by T-cells 1 (MART-1). The presence of premelanosomes and melanosome granules in the tumour cells are demonstrated by transmission electron microscopy, supports neural crest origin [8]. Ladanyi et al., demonstrated the expression of ER in SPPT and proposed that this tumour is hormone sensitive [9].
CD 99 immunoreactivity in SPPT has been reported recently in two studies on histopathology sections. CD 99 was demonstrated to have a characteristic paranuclear dot-like positivity in these studies [10,11]. They reported that this positivity pattern was quite classical of SPPT. In pancreatic endocrine tumours, it was either negative or had a membranous pattern of positivity [12]. In Ghosh et al., study, the studies on tissue sections had 100% of the SPPT positive for CD 99 [13].
According to the location of the tumour, distal pancreatectomy with splenectomy, pylorous preserving pancreatoduodenectomy, Whipple’s operation or enucleation can be performed consequently [14]. Furthermore, many reports had demonstrated less aggressive surgical procedures could be preferred for the treatment of SPPT [15]. Lashkarizadeh et al., detected the cystic mass in the area of the pancreatic head in a 20-year-old girl, they performed resection of the mass in the pancreatic head and pancreaticodeodenectomy (Whipple procedure) with jejunostomy tube placement [16]. Bektas reported a case of SPPT in a young women presented with unspecific complaints in the upper abdomen. They detected a mass in the area of the pancreatic head. They could resect only the tumour without a major procedure. In pancreatic head tumour if tumour is not large and the adjacent organs are not involved, this procedure is possible [17].
In our case the tumour was localized in the area of the uncinate process of pancreatic head, hence pylorus preserving pancreatico-duodenectomy was the preferred choice of surgery [18].
In the present case, the diagnosis was confirmed on histopathology study of the excised neoplasm. SPPT has distinctive FNA and histopathology appearance. Special investigations play a minor role, if any, in diagnosis but form the basis of histogenetic assertions [19] and hence the electron microscopy is of great value only in some cases in achieving a correct diagnosis of SPPT.
A high index of clinico-pathological suspicion is necessary to suspect and diagnose SPPT. This diagnosis should be borne in mind when young female patients present with a pancreatic mass. An accurate preoperative cytological diagnosis is very much possible, for a surgeon is better prepared to perform the appropriate surgery, to retain portion of the uninvolved pancreas if possible and prevents the development of subsequent diabetes mellitus [20].
Although, the histogenesis of this tumour is uncertain, SPPT is thought to originate from the pluripotent embryonic cells of the pancreas [15,21] or from ridges/ovarian anlage related cells, which were attached to the pancreatic tissue during early embryogenesis [22].
Conclusion
FNAC is a most valuable tool in the specific diagnosis of SPPT, cytomorphological recognition of this tumour is useful to diagnose these slow-growing, operable and potentially curable tumours of young women from the very much larger pool of usual pancreatic tumours with abysmal prognosis.
[1]. Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, Solid pseudopapillary tumour of the pancreas: a review of 553 cases in Chinese literatureWorld J Gastroenterol 2010 16(10):1209-14. [Google Scholar]
[2]. Frantz VK, Tumour of pancreas. In: Blumberg CW (ed)Atlas of tumour pathology. Series 1, Fascicles 27 and 28 1959 Washington, DC:32-33. [Google Scholar]
[3]. Milosevic B, Markovic R, Cvetkovic A, Solid and cystic pseudopapillary tumour of the pancreas: A Case ReportSrp Arh Celok Lek 2013 141(5-6):384-86. [Google Scholar]
[4]. Jayaram G, Chaturvedi KU, Jindal RK, Venugopal S, Kapoor R, Papillary cystic neoplasm of the pancreas report of a case diagnosed by fine needle aspiration cytologyActa Cytologica 1990 34(3):429-33. [Google Scholar]
[5]. Bondeson L, Bondeson AG, Genell S, Lindholm K, Thorstenson S, Aspiration cytology of a rare solid and papillary epithelial neoplasm of the pancreas. Light and electron microscopic study of a caseActa Cytol 1984 28:605-09. [Google Scholar]
[6]. Pailoor K, Kini H, Rau A, Kumar Y, Cytological diagnosis of a rare case of solid pseudopapillary neoplasm of the pancreasJ Cytol 2010 27(1):32-34. [Google Scholar]
[7]. Deshpande A, Munshi M, Cytology of papillary solid-cystic neoplasm of the pancreas presenting as an extrapancreatic mass. A case reportActa Cytologica 2004 48:1-7. [Google Scholar]
[8]. Salla C, Chatzipantelis P, Konstantinou P, Karoumpalis I, Pantazopoulou A, Dappola V, Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid pseudopapillary tumour of the pancreas: A case report and literature reviewWorld J Gastroenterol 2007 13:5158-63. [Google Scholar]
[9]. Ladanyi M, Mulay S, Arseneau J, Bettez P, Estrogen and progesterone receptor determination in the papillary cystic neoplasm of the pancreas. With immunohistochemical and ultrastructural observationsCancer 1987 60:1604-11. [Google Scholar]
[10]. Li L, Li J, Hao C, Zhang C, Mu K, Wang Y, Immunohistochemical evaluation of solid pseudopapillary tumours of the pancreas: The expression pattern of CD99 is highly uniqueCancer Lett 2011 310:9-14. [Google Scholar]
[11]. Guo Y, Yuan F, Deng H, Wang HF, Jin XL, Xiao JC, Paranuclear dot-like immunostaining for CD99: A unique staining pattern for diagnosing solid-pseudopapillary neoplasm of the pancreasAm J Surg Pathol 2011 35:799-806. [Google Scholar]
[12]. Goto A, Niki T, Terado Y, Fukushima J, Fukayama M, Prevalence of CD99 protein expression in pancreatic endocrine tumours(PETs)Histopathology 2004 45:384-92. [Google Scholar]
[13]. Ghosh R, Mallik S, Mathur Iyer V, CD 99 immunocytochemistry in solid pseudopapillary tumour of pancreas: A study on fine-needle aspiration cytology smearsJ Cytol 2013 30(3):151-55. [Google Scholar]
[14]. Lee W, Park Y, Choi J, Chi H, Kim B, Solid and papillary neoplasms of the pancreasYonsei Medical J 1996 37:131-41. [Google Scholar]
[15]. Rosai J, Pancreas and periampullary region. In: Rosai J, editorAckerman’s surgical pathology 2004 9th edSt. Louis, MissouriMosby:1061-114. [Google Scholar]
[16]. Lashkarizadeh MR, HayatbaKhsh M, Nikpour H, Savavi M, Lashkarizadeh M, Sattari Hosein, Solid-pseudopapillary tumour of the pancreas: a case report and Review of LiteratureIranian Journal of Pathology 2012 7(3):190-96. [Google Scholar]
[17]. Bektas H, Werner U, Kaaden S, Phillippou S, Kloppel G, Klempnauer J, Solid-pseudopapillary tumour of the pancreas- a rare and frequently misdiagnosed neoplasmLangenbecks Arch Surg 1999 384(1):39-43. [Google Scholar]
[18]. Igbinosa O, Pseudopapillary Tumour of the Pancreas. CASE REPORT An Algorithmic Approach. Saint Peter’s University HospitalNew Brunswick, NJUSA- http://www.joplink.net [Google Scholar]
[19]. Orell SR, Sterrett GF, Whitaker D. Fine needle aspiration cytology 4th edition, Pg. no. 324 [Google Scholar]
[20]. Mehta N, Modi L, Patel T, Shah M, Study of cytomorphology of solid pseudopapillary tumour of pancreas and its differential diagnosisJ Cytol 2010 27(4):118-22. [Google Scholar]
[21]. Jayaram N, Prasad S, Tambwekar S, Ramprasad AV, Papillary solid – cystic epithelial neoplasm of pancreas. Report of a case diagnosed by FNACJournal of Cytology 2000 17(2):115-19. [Google Scholar]
[22]. Rebhandl W, Felberbauer FX, Puig S, Kurosh P, Hochschorner S, Barlan M, Solid-pseudopapillary tumour of the pancreas (Frantz tumour) in children: report of four cases and review of the literatureJ Surg Oncol 2001 76:289-96. [Google Scholar]