The aim of the present study was to assess the various clinical aspects of CVT patients presenting to our hospital in a prospective manner.
Materials and Methods
This was a prospective study carried out from September 2012 to July 2014 in Department of Internal Medicine, Jipmer, Puducherry. Ethics committee approval was obtained. The study included three groups. In the first group, we included the patients who were 13 years of age or above and diagnosed of CVT by imaging. Second group consisted of one control (age and duration of puerperium matched) for each case of puerperal CVT included in first group. In the third group, we included non-puerperal patients who were having symptoms and signs suggestive of CVT but were proven to have normal venous sinuses by imaging. The second and third groups were defined only for evaluation of D-dimer. So, the patients with conditions that can lead to elevation of D-dimer were excluded. Detailed history was obtained and neurological examination was done. The patients were assessed with the Modified Rankin score (mRS) and Barthel index (BI) on Day 1, at discharge and after three months. Thrombophilia workup was done only when indicated due to financial constraints. Sample for D-dimer was collected within 24 hours of diagnosis. D-dimer was assessed by a rapid semi- quantitative latex agglutination assay (STAGO, D-DI TEST). Test was repeated with serial dilutions and maximum range of D-dimer was defined for each patient. The anatomic location and extent of infarct or haemorrhage was assessed by neuroimaging. The patients were reviewed after one month and three months. The categorical variables among the subgroups were compared using Fisher’s-exact test. The means and medians of the continuous variables were compared using Mann-Whitney test. The diagnostic performance characteristics of D-dimer were assessed with neuroimaging. A ROC curve was plotted to define the level of D-dimer in puerperium using the available D-dimer levels in puerperal CVT patients and the matched controls. All the statistical analysis was performed using GraphPad InStat 3.12.
Clinical profile of patients.
| Parameter | Number (n=80) | Percentage |
|---|
| Headache | 77 | 96.25% |
| Seizures | 51 | 63.75% |
| Vomiting | 44 | 55% |
| Motor weakness | 32 | 40% |
| Altered sensorium | 22 | 27.5% |
| Speech disturbances | 12 | 15% |
| Visual disturbances | 7 | 8.75% |
| Clinical syndrome |
| Composite neurological deficit with seizure | 30 | 37.5% |
| Composite neurological deficit without seizure | 12 | 15% |
| Seizure | 21 | 26.25% |
| Isolated intracranial hypertension* | 14 | 17.5% |
| Isolated headache | 3 | 3.75% |
| Acute (less than 48 hours of symptoms) | 20 | 25% |
| Subacute (symptoms for 48 hours to 30 days) | 55 | 68.75% |
| Chronic (symptoms lasting more than 30 days) | 5 | 6.25% |
*Isolated Intracranial Hypertension syndrome was defined as {headache, vomiting, and papilloedema, visual loss or sixth nerve paresis, without focal neurological deficit (FND)}.
Eighty patients were included in the study. Of them, 44 were women and 36 were men (F: M=1.2:1). The mean age of the patients was 29.5±9.68 (14-55) years. Headache occurred in 77 patients. Of them 60 patients (77.3%) presented with headache of less than ten days, while only six (7.7%) presented with headache of more than a month duration. Most common clinical syndrome was headache with neurological deficit (altered sensorium, weakness, seizure, speech disturbance or visual disturbance). Seizures occurred in 51 patients. Of them, generalized seizures occurred in 33(64.7%) while focal seizures occurred in 16 (31.3%). Status epilepticus was noted only in two (3.9%) patients Thirty one (39%) presented with hemiparesis. Only one patient presented with paraparesis. Most of the patients presented with headache that preceded the neurological deficit or presentation to hospital. Only three patients presented directly with neurological deficit. Interval between headache and neurological deficit was noted in 60 patients with neurological deficit. The mean interval was 6.41±10.6 days (1 day to 2 months). Papilloedema was noted in 54(67.5%) of patients.
One or other causes of CVT were identified in 58 patients while in 10 patients the cause was unidentified. The causes of CVT are mentioned in [Table/Fig-2]. In the other 12 patients we found factors that can contribute to development of CVT. They were anaemia especially Iron Deficiency Anaemia (5/6), Smokers Polycythemia, chicken pox. Additional factors that contribute to development of CVT along with main cause were found in 25 patients. Iron deficiency anaemia was the most common additional factor in combination with main causes noted in 18 patients. Other additional factors included alcoholism and smoking.
| Causes |
|---|
| Pregnancy | 26 | 32.5% |
| Systemic sepsis | 10 | 12.5% |
| Dehydration | 7 | 8.75% |
| Polycythemia Vera | 2 | 2.5% |
| Hyperhomocysteinemia | 3 | 3.75% |
| Connective tissue disorders | 2 | 2.5% |
| Local infection | 3 | 3.75% |
| Malignancy | 1 | 1.25% |
| Drugs | 4 | 5% |
| Unidentified | 10 | 12.5% |
| Anaemia | 6 | 7.5% |
| Smokers Polycythemia | 3 | 3.75% |
| Chicken pox | 3 | 3.75% |
| Iron deficiency anaemia | 18 | 22.5% |
| Polycythemia | 2 | 2.5% |
| Antiphospholipid antibody syndrome | 1 | 1.25% |
Laboratory and imaging parameters.
| Laboratory parameters | Number of patients | Percentage |
|---|
| Anaemia | 46 (57.5%) | 57.5% |
| Iron deficiency anaemia | 23 (28.75%) | 28.75% |
| Normocytic/macrocytic anaemia | 23 (28.75%) | 28.75% |
| Polycythemia | 7 (8.75%) | 8.75% |
| Thrombosed sinus |
| Superior sagittal | 62 | 77.5% |
| Transverse | 61 | 76.25% |
| Sigmoid | 43 | 53.75% |
| Straight | 4 | 5% |
| Inferior sagittal | 7 | 8.75% |
| Cavernous | 1 | 1.2% |
| Superior petrosal | 1 | 1.2% |
| Sigmoid sinus | 2 | 2.5% |
Anaemia (Hb in male <13, non-pregnant female <12 pregnant female<11) was noted in 46 patients. Of the total, 23 patients (21 females and 2 males) were found to have iron deficiency anaemia (microcytic hypochromic RBCS and low ferritin). Sepsis (neutrophilic leukocytosis with toxic change) was noted in 12 patients. Polycythemia (Hb>18.5g/dl) was noted in seven patients. CT Venography was done in 73 patients while MRV was done in seven patients. Superior sagittal and transverse sinuses were the most common sinuses to be affected and were involved in more than 2/3rd of the patients. Deep sinuses (transverse or sigmoid or petrosal or straight sinus) were involved in 65 patients. Cerebral oedema was noted in 59(74%) patients. Infarct was noted in 29(36.2%) patients while haemorrhagic infarction was noted in 18(22.5%) patients. Only three (3.75%) patients presented with haemorrhage alone.
The mean hospital stay was 7±5.5 days ranging from a day to a month. Seventeen (21%) patients expired. All the 17 deaths occurred in hospital. Decompression was done in seven patients. Of 63 patients, only one patient lost to follow-up after discharge. Only three patients were dependent (mRS≥3) at the end of follow-up.
Evaluation of D-Dimer
D-dimer was done in 65 out of 80 patients of CVT. D-dimer was not done in patients with sepsis(12), TB meningitis(1), SLE(1) and malignancy(1). D-dimer was also done in 15 patients included in third group.
The sensitivity and specificity of D-dimer for diagnosing CVT was 84.62% and 80% respectively in this group. The Positive Predictive Value (PPV) of D-dimer for CVT was 94.8% while Negative Predictive Value (NPV) was 54.5%. Out of 80 patients, 62 patients presented within seven days. Out of them, D-dimer was done in 48 patients and was positive in 45 patients. The sensitivity of D-dimer in these patients was 93.8%.
D-dimer was done in 24 puerperal CVT and 24 controls. The ROC curve [Table/Fig-4] for range of D-dimer and puerperal CVT defined 1000-2000 as the cut off for diagnosis with a sensitivity and specificity of 75% and 50%. The PPV of D-dimer in this group was 60% and NPV was 66%. The p-value for D-dimer test was 0.13, implying no statistically significant difference between puerperal CVT cases and matched controls.
ROC Curve for D-Dimer in puerperium.
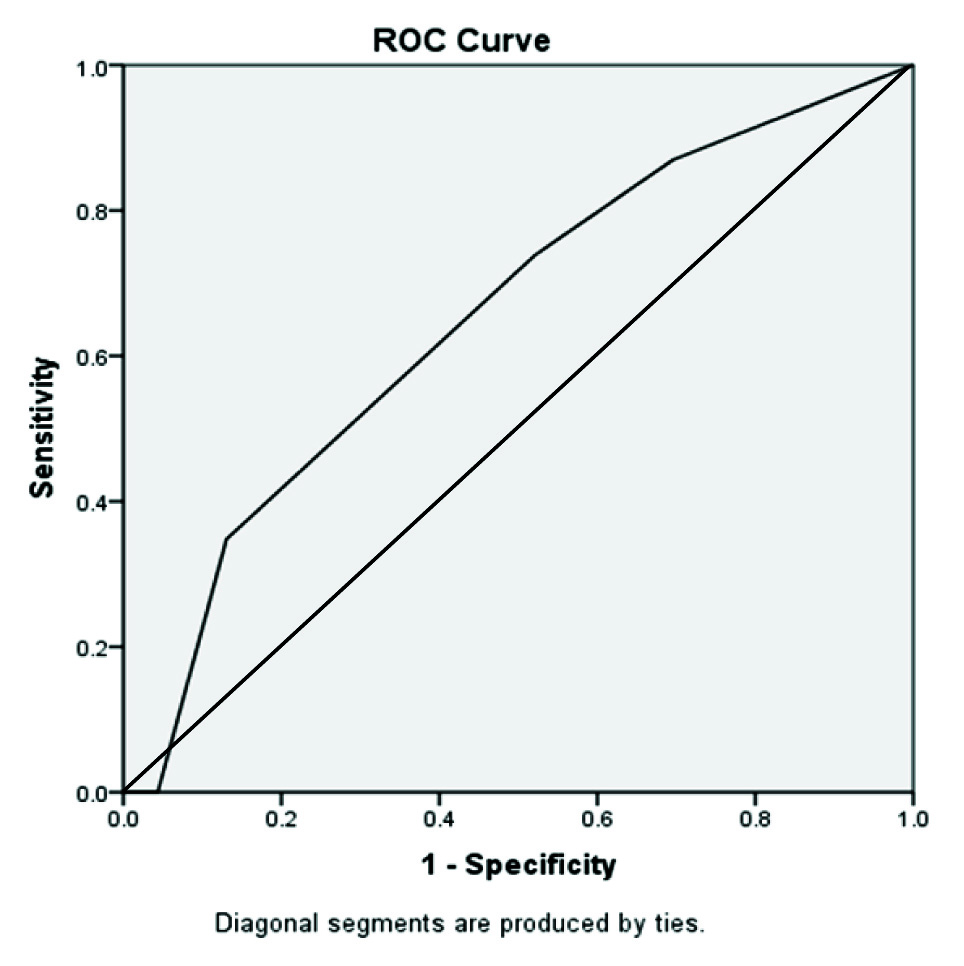
D-dimer levels in patients were compared with the neurological imaging findings in the patients. The extent of infarct or haemorrhage was compared with the range of D-dimer and correlated using Spearman’s correlation. The Spearman’s Correlation Coefficient was found to be +0.40 implying weak correlation.
Out of 80 patients 17 expired, 13(76%) patients presented with headache with duration of less than seven days. The variables that had statistical significance between mortality and survival groups were presence of altered sensorium at presentation (100% vs 31% p<0.0001), presence of sepsis {(83% vs 3% RR 8.09 (3.8-17.07) p<0.0001)}. Patients in mortality group had more incidences of altered sensorium at presentation and high mRS and low BI at admission as compared to the survival group {(100% vs 30% p<0.0001)}. Similarly, this group of patients had more frequent sepsis and more number of sinuses involved as compared to patients who were in survival group.
CVT patients with sepsis had an increased risk of presentation with altered sensorium as compared to patients without sepsis {(91.6% vs 16%, RR 29(3.97-211) p<0.0001)}. Intracranial bleed after anticoagulation occurred in four (33%) of sepsis patients while it did not occur in patients without sepsis. Patients with sepsis had increased mortality as compared to patients without sepsis {(83.3% vs 10.2% RR (3.83-17.07), p<0.001}.
D-dimer was negative in 10 CVT patients. These groups of patients presented with headache of long duration as compared to D-dimer positive patients (5.17±3.06 vs. 95.3±143, p<0.0001). D-dimer negative patients had very good prognosis and none of the patient expired in this group as compared to six deaths in D-dimer positive group.
Discussion
In this prospective study including 80 patients of CVT, the clinical features, neuroimaging, prognosis and D-dimer were analysed. Out of 80 patients, 44 patients were females and males were 36(F: M=1.2:1). This was in contrast to previous studies [3,4] in which females constituted more than 70% of the cases. However, these studies were done more than a decade back. The two large studies from India were done in the last decade at Hyderabad [5] and Mumbai [6] done by Narayanan D et al., including 428 patients and Navin Pai et al., including 612 patients respectively had M: F ratio of 1.17:1 and 1.5:1 showing male preponderance. Our study tends to show a similar trend but due to the lesser number of patients included could not show the male preponderance as is being thought now. The mean age of our patients was 29.5±9.68 years, which was similar to most studies [5,6]. We classified our patients into four clinical syndromes for prognostic purpose and also because of their mode of presentation. Sixteen (25%) out of 63 patients with neurological deficit expired while only 1(5%) out of 17 with isolated intracranial tension or isolated headache expired.
The causes of CVT are diverse. Most common cause of CVT we found was puerperium (33%). Earlier studies [7] showed puerperium as a major cause (60%) of CVT; however present studies [4,5] shows a decreasing trend. International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) showed mortality of 12.3% and it was 9.8% in study from Nizam’s Institute of Medical sciences (NIMS). The independent risk factors proposed for increased risk of CVT in puerperium were caesarean section (OR 3.1), intercurrent infection (OR 3.45), maternal hypertension (OR 1.9), increasing maternal age (OR 2.5), and presence of several other co-morbidities including hyperemesis (OR 14.9) [8]. The changes in the trends of these risk factors could also contribute to decreasing trends of CVT in puerperium. Out of 25 postpartum CVT patients, three (12%) underwent caesarean section and none had hypertension. The mean age of patients with pregnancy related CVT was 29.8 (19-33) years.
Systemic Sepsis constituted to one of the second common causes of CVT in our study. We defined systemic sepsis as patients who are having documented fever in hospital, neutrophilic preponderance and toxic change. Lumbar puncture was done in five patients without midline shift and was normal. In ISCVT [4], infections constituted to 12.3% of CVT cases while in Indian studies the sepsis was cause of CVT in 6.5% people in a study on hereditary thrombophilia in CVT [6] and 2.1% in NIMS study [5]. In our study systemic sepsis was noted in 10 patients (12.5%). The activation of coagulation pathway, platelets and endothelium frequently occur in patients with severe sepsis or infections leading to thrombosis [9].
Anaemia was noted in 46(57.5%) of our patients with CVT. In majority (40 patients) there were other associated conditions. Isolated anaemia occurred in six patients and all had severe anaemia (Hb<9g/dl). In these six patients, Iron deficiency anaemia occurred in four patients while megaloblastic anaemia due to B12 deficiency occurred in one patient. In one patient we could not find the cause of anaemia. The association between anaemia and CVT was less studied. The first study [10] included 121 CVT patients and 120 healthy controls. Severe anaemia (<9g/dl) was found to be independently associated with CVT (OR 1.10, 95% CI 1.01-2.22, p <0.05).
Iron deficiency anaemia (IDA) was noted in 23 patients (F: M=21:2). Among them 19 had other predisposing condition to develop CVT (puerperium 17, chicken pox 1, and sepsis 1). The other four patients had only IDA as the cause of CVT. Out of these four patients only one had thrombocytosis. Iron deficiency anaemia as a cause of CVT is scarcely studied and most of evidence is from case reports [11]. The predisposition to CVT could be either due to associated thrombocytosis [12] or the alteration of pattern of blood flow within the vessels due to decreased deformability and increased viscosity i.e. thickness of microcytic RBC [13]. IDA has to be evaluated as a cause of CVT.
Polycythemia was the cause of CVT in seven patients (8.75%) in our study. Polycythemia was the cause of CVT in 8(1.8%) of the patients in the NIMS study [5]. All these patients had Hb>18.5g/dl. Out of the seven patients two patients were diagnose to have polycythemia vera. Four of the patients with polycythemia were smokers and had significant pack years. These patients had low oxygen saturation on room air with pulse-oximetry as well as arterial blood gas analysis. All these patients also had elevated erythropoietin levels. Smokers’ Polycythemia as a cause of CVT till now is rarely reported [14]. The two mechanisms attributed are elevated red cell count production along with decrease in plasma volume [15].
Following chickenpox infection CVT is being increasingly reported [16,17]. We considered varicella infection as a cause of CVT in three patients. All of them presented with headache after varicella eruption have subsided. Varicella Immunoglobulin (VZIg) was done in two patients and was positive with a reduced serum to Cerebrospinal Fluid (CSF) ratio. The cause of thrombosis after varicella infection was supposed to transaxonal migration of varicella infection or due to transient protein C deficiency [18].
We did D-dimer in our patients to assess role of D-dimer in early diagnosis of CVT. The sample for D-dimer was collected on day 2 of hospitalization. Most patients were on anticoagulation at the time of collection of sample. The sensitivity and specificity were in correlating with previous studies [19,20]. The poor negative predictive value in our study could be due to lack of adequate controls in our study. Due to economic constraints, we could not do quantitative D-dimer levels and so we carried out a semi quantitative assay using serial dilution method.
Our study is the only study which compared D-dimer levels among puerperal patients with CVT and puerperal controls. D-dimer did not show good correlation with number of sinuses involved. This was in similar to a previous study done in India [21] in which D-dimer level was inversely related to duration of symptoms and did not correlate well with thrombus burden.
All except one of the patients received anticoagulation in our study. We did not collect data on types of anticoagulation and protocols followed in hospital. Anticoagulation related bleed occurred in four patients. All these patients had sepsis.
Mortality was 21.25% and higher then usually reported. The predictors for death and dependence in previous studies were male sex, altered sensorium, age>37 years, deep venous system thrombosis, ICH and CNS infection [3]. As compared to ISCVT [3] in which 39% of the patients presented with isolated intracranial hypertension (IIH), in our study only 17% of the patients are in IIH group. This could be the reason behind high mortality in our study. Also, our study had 12 patients (15%) of the patients with sepsis. The prognostic factors for bad prognosis in our study were sepsis, extent of sinus involvement and altered sensorium (high mRS and low BI) at presentation.
Conclusion
Cerebral venous thrombosis is a disease with equal preponderance among both genders affecting mostly young individuals. Most patients present with a headache and neurological deficit. Puerperium still contributes to majority of the cases. Iron deficiency anaemia needs to be evaluated as a contributing factor for incidence of CVT. CVT has to be kept in mind while treating patients presenting with stroke after varicella infection. The patients who survive have a good prognosis and near complete recovery. D-dimer is not useful in puerperal female with CVT. Positive D-dimer will strengthen the suspicion of CVT in a patients with acute headache followed by a neurological deficit. D-dimer negative patients have a good prognosis.
*Isolated Intracranial Hypertension syndrome was defined as {headache, vomiting, and papilloedema, visual loss or sixth nerve paresis, without focal neurological deficit (FND)}.