Non-Secretory Myeloma, Diagnosed on Renal Biopsy as Cast Nephropathy
Sumit Grover1, Pavneet Kaur Selhi2, Neena Sood3, Jasvinder Singh Sandhu4, Harpreet Kaur5
1 Assistant Professor, Department of Pathology, Dayanand Medical College and Hospital, Ludhiana, Punjab, India.
2 Professor, Department of Pathology, Dayanand Medical College and Hospital, Ludhiana, Punjab, India.
3 Professor and Head, Department of Pathology, Dayanand Medical College and Hospital, Ludhiana, Punjab, India.
4 Professor and Head, Department of Nephrology, Dayanand Medical College and Hospital, Ludhiana, Punjab, India.
5 Professor, Department of Pathology, Dayanand Medical College and Hospital, Ludhiana, Punjab, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sumit Grover, Assistant Professor, Department of Pathology, Dayanand Medical College and Hospital, Tagore Nagar, Ludhiana, Punjab- 141001, India.
E-mail: sumitgrover1204@gmail.com
Multiple myeloma is a disorder of plasma cells which can involve kidneys in the form of cast nephropathy. Neoplastic plasma cells produce either complete immunoglobulins or fragments of immunoglobulins leading to a monoclonal spike in the serum and/or Bence Jones proteinuria. Very few patients present as non-secretory myeloma when no immunoglobulins (M spike) are produced or only light chains are secreted which can only be detected in urine. Acute renal failure due to cast nephropathy can rarely be the first presentation of multiple myeloma. We here in report a case in which primary diagnosis of multiple myeloma was made on renal biopsy due to its characteristic histomorphology. The diagnosis was later on supported by presence of neoplastic plasma cells in the aspirate and biopsy of bone marrow.
Fractured casts, Kidney biopsy, Plasma cell dyscrasia, Protein electrophoresis, Renal failure
Case Report
A 52-year-old female patient presented with pedal and periorbital oedema, loss of weight and appetite with decreased urine output in medicine outpatient department. She gave a past history of joint pains involving proximal interphalangeal joints, metacarpophalangeal joints, wrist joints and bilateral shoulder joints along with Raynaud’s phenomenon and gangrene of right and left index finger 12 years back with partial shedding of digits. On investigation she was found to have a serum creatinine of 9.08 mg/dL and hence diagnosed rapidly progressive renal failure of unknown cause. Other biochemistry investigations revealed serum calcium 8.2 mg/dL, phosphate of 7.9 mg/dL, uric acid 8.3 mg/dL, albumin 3.8 g/dL, globulins 3.4 g/dL, blood urea nitrogen (BUN) 215 mg/dL, total protein 7.2 g/dL, Na 121 mEq/l; K 4.5 mEq/l and alkaline phosphatase 83 KA units. 24 hour urine total protein excretion was 1.9 g with 10-12 pus cells/hpf, however, no RBC were seen. Renal ultrasound showed bilateral normal kidneys. A clinical diagnosis of Scleroderma renal crisis was made and a renal biopsy performed.
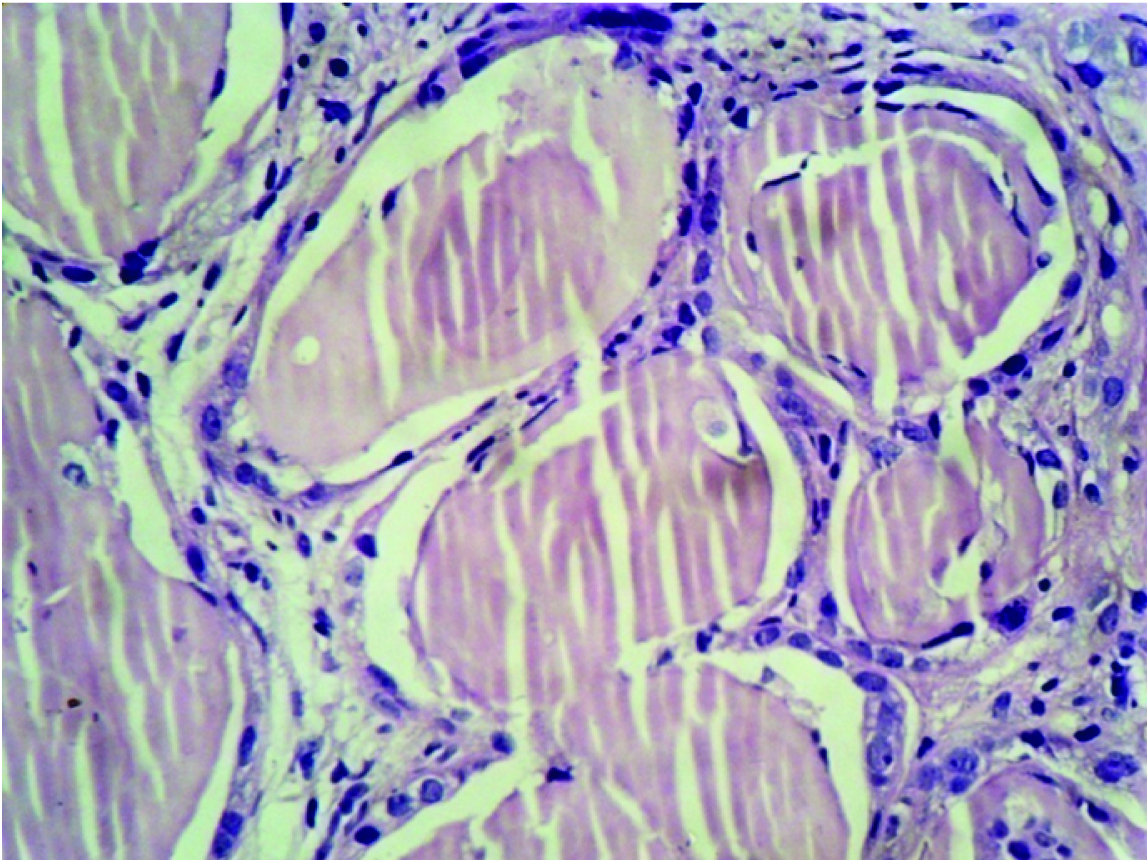
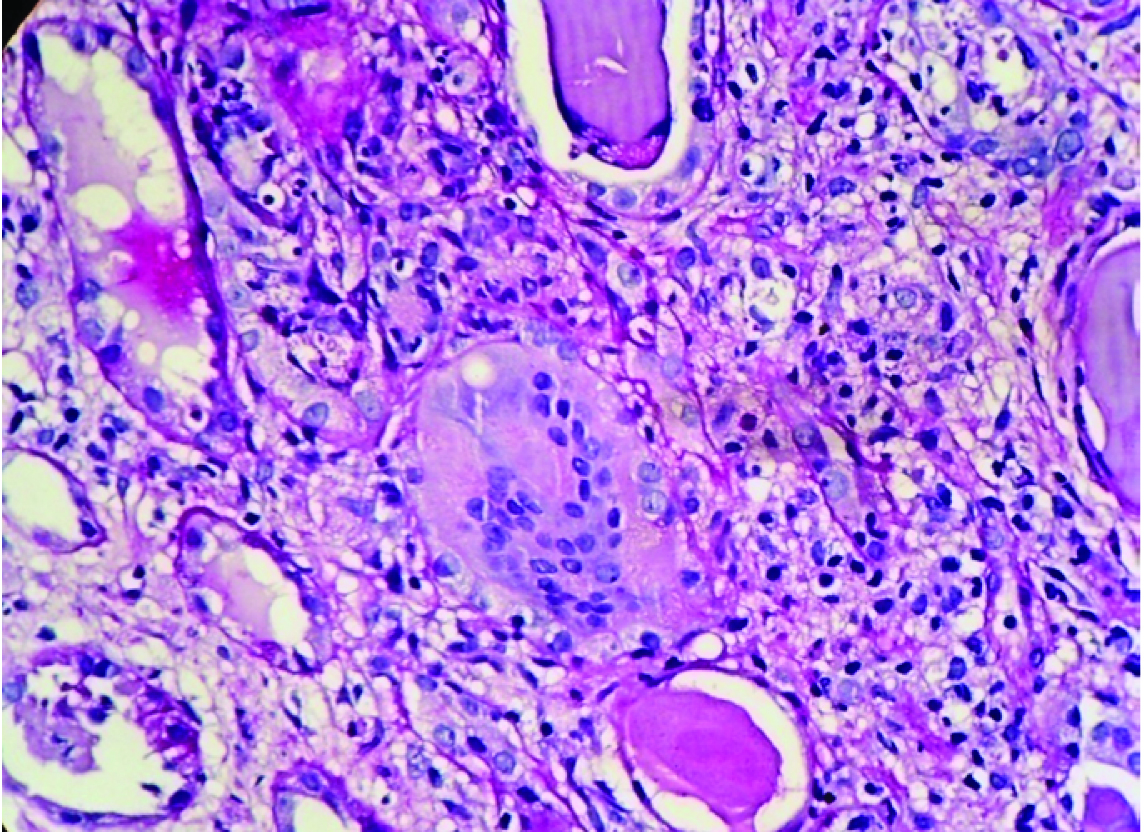
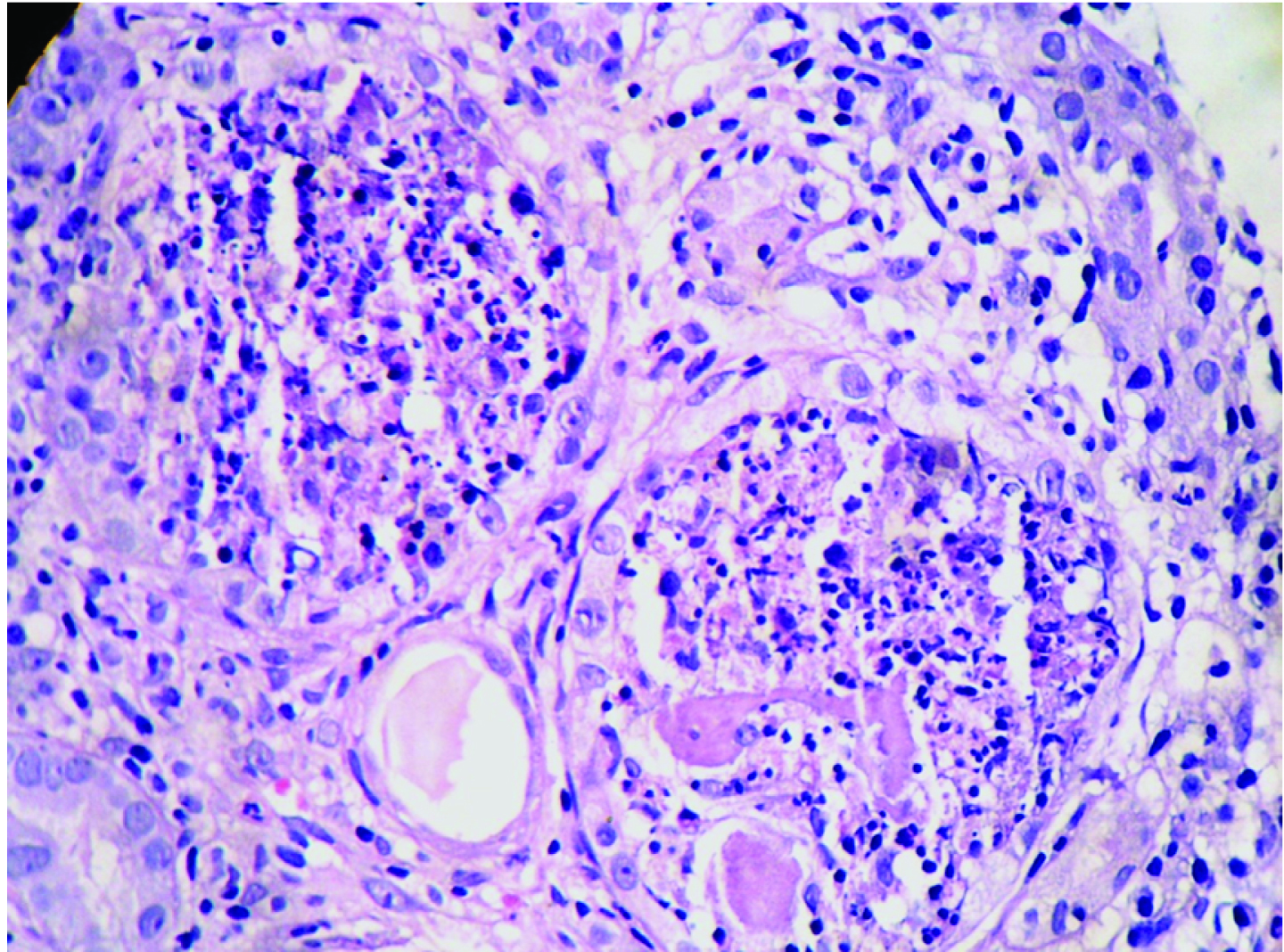
Renal biopsy was adequate and composed of 21 glomeruli, all of which were histologically unremarkable. Patchy tubular atrophy was evident with dilatation of few which showed pink eosinophilic fractured casts surrounded by multinucleated giant cells at places [Table/Fig-1,2] and accompanied by moderate mixed interstitial infiltrate consisting of lymphocytes, histiocytes and neutrophils [Table/Fig-3]. Blood vessels showed no specific pathology. No fibrin thrombi/ infarcted glomeruli or tubule/fibrinoid necrosis/glomerulosclerosis/ fibrointimal thickening of arteries/ onion skin lesions were seen. Congo red stain for amyloid was negative. Immunofluorescence (IF) performed using antisera to human IgG, IgA, IgM, C3 and fibrinogen showed tubular casts staining positive for IgG.
Dilated Renal tubules filled with pink eosinophilic fractured casts (H&E X400).
Giant cell reaction around casts with interstitium showing lympho-mononuclear infiltrate (PAS X400).
Tubules showing neutrophilic infiltrate around casts with reactive epithelium (H&E X400).
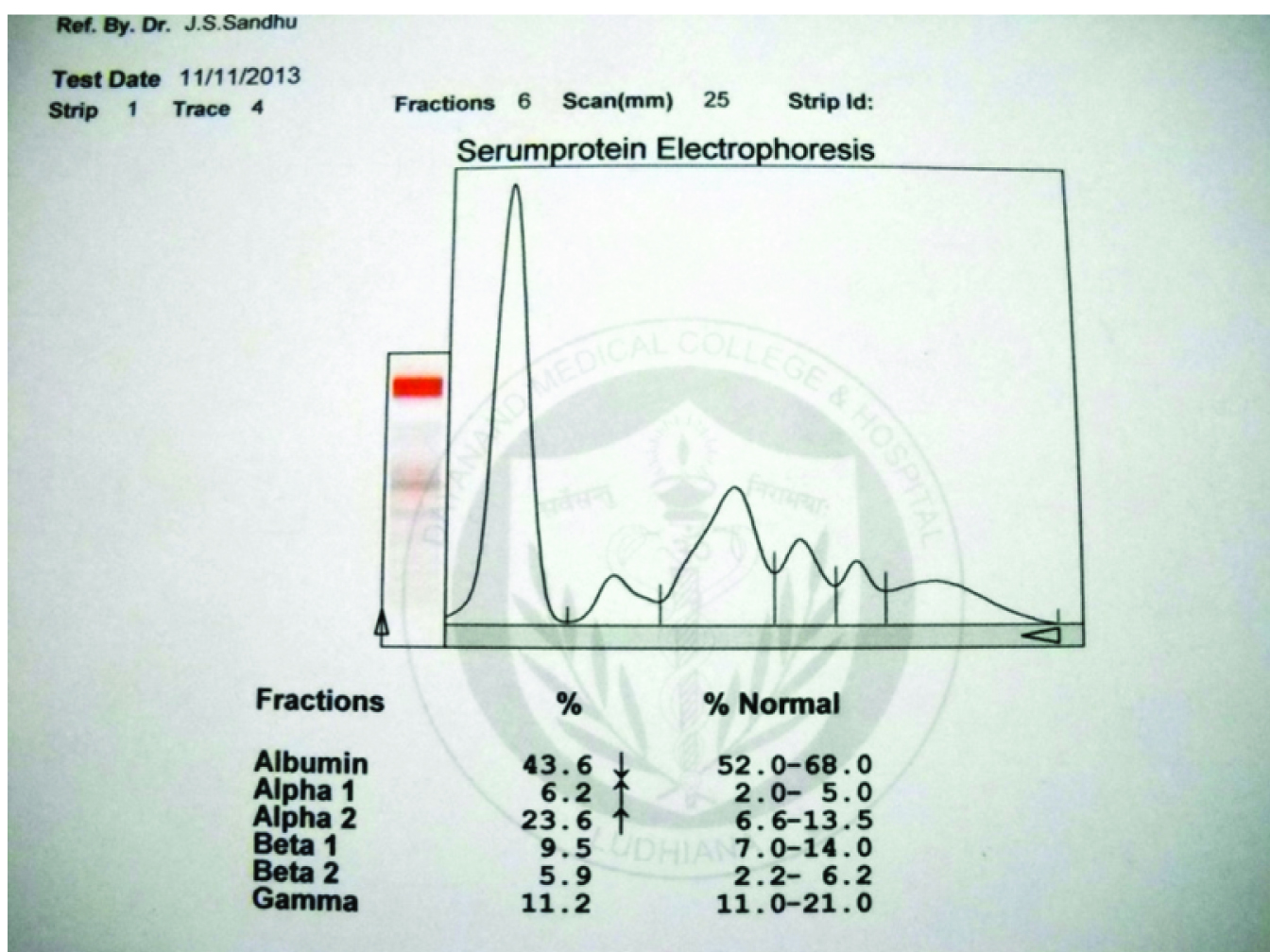
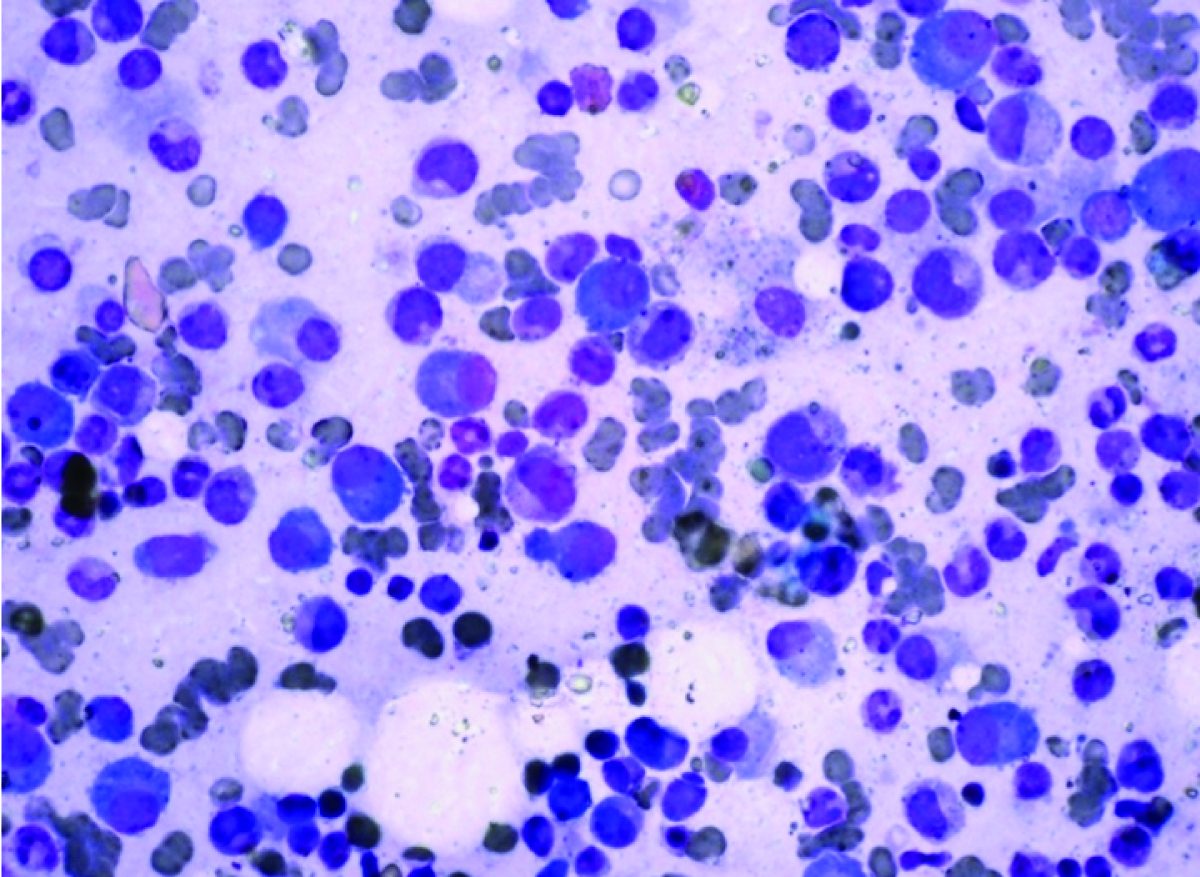
Further, a battery of investigations i.e. serum protein electrophoresis and aspiration of bone marrow was performed. SPE showed no monoclonal spike [Table/Fig-4]. Urine for Bence-Jones protein was consistently negative. Bone marrow showed plasma cells to the tune of 35% of all nucleated cells [Table/Fig-5]. Thus, a diagnosis of Plasma cell dyscrasia- Multiple myeloma was made.
Serum protein electrophoresis curve without a peak in gama or beta region.
Bone marrow aspirate showing numerous plasma cells among other haematopoietic cells (MGG X400).
Discussion
Multiple myeloma accounts for approximately 10% of all haematologic neoplasms [1]. Multiple myeloma is the most advanced manifestation of plasma cell dyscrasia which presents typically as multiple lytic (punched out) bone lesions associated with an increase in the number of bone marrow plasma cells (in a range of 15% to 20%). Either complete immunoglobulin (Ig) or fragments of Ig are produced by the neoplastic plasma cells leading to a monoclonal spike in the serum and/or BJ proteinuria. 1-5% of all cases may not show the band which are called ’’Non-Secretory Myeloma (NSMM)’’ [2]. Myeloma commonly involves kidneys in form of Cast Nephropathy which typically presents as acute renal deterioration or frank renal failure [3–5]. Based on Immunohistochemistry (IHC), NSMM are divided into non-producers (15% cases) and producers (85% cases) [6]. Producers possess a secretion defect leading to lack of Ig in blood but may show evidence of Ig in plasma cells by IHC. NSMM must also be differentiated form “free light chain only” myeloma needing free light chain assay for diagnosis [7].
Renal insufficiency frequently complicates secretory myeloma and is the second most common cause of death after infection in these patients [1,5]. The excessively produced Ig free light chains are excreted by kidneys and reabsorbed in proximal tubules. When the threshold of proximal tubule for reabsorption of light chains is exceeded or damage to the proximal tubules impairs reabsorption, the light chains are delivered to the distal portion of the nephron and may produce obstructing tubular casts, leading to a renal condition called cast nephropathy [8]. These casts are protein-rich, irregular, angulated with fracture planes and alamellate internal appearance in the distal tubules. Casts are typically fuschinophilic with Masson’s trichrome, brightly eosinophilic, PAS negative and occasionally Congo red positive. Interstitium shows an inflammatory reaction, predominantly with mononuclear inflammatory cells and sometimes eosinophils because of rupture of the tubular basement membranes and spillage of the cast contents, including Tamm-Horsfall protein and irreversible interstitial damage and fibrosis [9]. Tubules with casts show reactive epithelial cells with presence of multinucleate giant cells. These obstructing tubular casts and interstitial fibrosis ultimately lead to renal shutdown.
Achieving an early reduction in serum monoclonal free light chain in (FLC) levels allows renal recovery in a high proportion of secretory myeloma patients which can be achieved by effective chemotherapy [10,11]. The immunomodulatory and bortezomib-based regimens, along with high-dose dexamethasone rapidly reduce clonal plasma cells and renal tubulointerstitial inflammation [12]. Persistent elevation of FLC may cause rapid progression of interstitial fibrosis leading to irreversible renal impairment [13].
The clinical behaviour differs in patients presenting with NSMM from those presenting as oligosecretory myeloma at the outset or as secretory myeloma which later on converts to non-secretory myeoma at relapse. The latter patients typically have high-risk myeloma, genomic instability, and rapid clonal evolution. As NSMM patients, present with cytopenias or bone disease, positron emission tomography (PET)/CT imaging along with marrow plasmacytosis, was previously used to assess the level of disease response [7,14]. But researchers in recent studies found assessment of disease burden using routine marrow histology and routine flow cytometry to be inaccurate, due to patchy nature of marrow involvement and issues with sampling [7,15]. They found multiparameter flowcytometry as the appropriate method with good predictive and prognostic implications to evaluate minimal residual disease post treatment [16].
Prognosis wise treatment with three drug combinations involving proteasome inhibitors together with immunomodulatory agents like lenalidomide, bortezomib, and dexamethasone (RVD) or or bortezomib, thalidomide, and dexamethasone (VTD), or proteasome inhibitors in combination with alkylating agents (bortezomib, cyclophosphamide, and dexamethasone (VCD) showed the same 3-year overall survival and progression free survival in both secretory as well as non-secretory myeloma [7,17–20].
Conclusion
The diagnosis as well as follow-up of non-secretory myeloma needs a combination approach including thorough initial evaluation along with additional studies like Multiparameter Flocytometry in conjunction with PET/CT scanning. However further large scale studies are needed to evaluate the response and prognosis of NSMM versus secretory myeloma. We report this case as NSMM has rarely been diagnosed on renal biopsy in a patient who presents with acute renal failure of unknown origin. In this case the characteristic histomorphology on renal biopsy aided in the diagnosis of cast nephropathy and thereby multiple myeloma.
[1]. Kyle RA, Multiple myeloma: Review of 869 casesMayo Clin Proc 1975 50:29 [Google Scholar]
[2]. Bladà J, Kyle RA, Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemiaHaematol Oncol Clin North Am 1999 13:1259 [Google Scholar]
[3]. Start DA, Silva FG, Davis LD, D’Agati V, Pirani CL, Myeloma cast nephropathy: immunohistochemical and lectin studiesMod Pathol 1988 1:336-47. [Google Scholar]
[4]. Cohen AH, The kidney in plasma cell dyscrasias: Bence-Jones cast nephropathy and light chain deposit diseaseAm J Kidney Dis 1998 32:529 [Google Scholar]
[5]. Niesvizky R, Siegel D, Michaeli J, Biology and treatment of multiple myelomaBlood Rev 1993 7:24 [Google Scholar]
[6]. Middela S, Kanse P, Nonsecretory multiple myelomaIndian J Orthop 2009 43:408-11. [Google Scholar]
[7]. Lonial S, Kaufman JL, Non-secretory myeloma: a clinician’s guide>Oncology (Williston Park) 2013 27:924-28.:930 [Google Scholar]
[8]. Sanders PW, Booker BB, Pathobiology of cast nephropathy from human Bence Jones proteinsJ Clin Invest 1992 89:630 [Google Scholar]
[9]. Thomas DB, Davies M, Peters JR, Williams JD, Tamm Horsfall protein binds to a single class of carbohydrate specific receptors on human neutrophilsKidney Int 1993 44:423-29. [Google Scholar]
[10]. Leung N, Gertz MA, Zeldenrust SR, Rajkumar SV, Dispenzieri A, Fervenza FC, Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chainsKidney Int 2008 73:1282-88. [Google Scholar]
[11]. Hutchison CA, Bradwell AR, Cook M, Basnayake K, Basu S, Harding S, Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysisClin J Am Soc Nephrol 2009 4:745-54. [Google Scholar]
[12]. Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working GroupJ Clin Oncol 2010 28:4976-84. [Google Scholar]
[13]. Basnayake K, Cheung CK, Sheaff M, Fuggle W, Kamel D, Nakoinz S, Differential progression of renal scarring and determinants of late renal recovery in sustained dialysis dependent acute kidney injury secondary to myeloma kidneyJ Clin Pathol 2010 63:884-87. [Google Scholar]
[14]. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working GroupBr J Haematol 2003 121:749-57. [Google Scholar]
[15]. Paiva B, Martinez-Lopez J, Vidriales MB, Mateos MV, Montalban MA, Fernandez-Redondo E, Comparison of immuno fixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myelomaJ Clin Oncol 2011 29:1627-33. [Google Scholar]
[16]. Paiva B, Vidriales MB, Cervero J, Mateo G, Perez JJ, Montalban MA, Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantationBlood 2008 112:4017-23. [Google Scholar]
[17]. Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myelomaBlood 2010 116(5):679-86. [Google Scholar]
[18]. Kaufman JL, Nooka A, Vrana M, Gleason C, Heffner LT, Lonial S, Bortezomib, thalidomide, and dexamethasone as induction therapy for patients with symptomatic multiple myeloma: a retrospective studyCancer 2010 116:3143-51. [Google Scholar]
[19]. Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 studyLancet 2010 18:2075-85. [Google Scholar]
[20]. Kumar S, Perez WS, Zhang MJ, Ballen K, Bashey A, To LB, Bredeson CN, Comparable outcomes in nonsecretory and secretory multiple myeloma after autologous stem cell transplantationBiol Blood Marrow Transplant 2008 14:1134-40. [Google Scholar]