Ventilator-Associated Pneumonia (VAP) refers to nosocomial pneumonia occurring 48 hours or more after initiation of mechanical ventilation (MV) [1]. VAP is the most common Hospital-Associated Infection (HAI) among adult patients in Intensive Care Units (ICUs), with frequencies ranging from 15-45% [2]. Moreover, it is the second most common HAI after blood stream infection in the paediatric age group, accounting for about 20% of all HAIs in the paediatric intensive care units (PICUs) and has a rate of 2.9-21.6 per 1000 ventilator days [3]. VAP is associated with increased hospital morbidity; mortality; duration of hospitalization by an average of 7-9 days per patient; and health care costs [4–7].
The incidence rates of VAP are higher in developing countries with limited resources [8]. In Egypt, a study of device-associated infection rates in the PICUs in a number of hospitals has showed that the overall rate of HAIs was 24.5% and that of VAP was 31.8 per 1000 ventilator days [9].
The onset of VAP can be divided into: early which occurs 48 to 96h after intubation and late which occurs more than 96h after intubation. Early and late VAP differ in their pathogenesis, microorganisms responsible, antibiotic sensitivity, outcome and treatment [10]. The pathogenesis of VAP involves bacterial invasion of the pulmonary parenchyma in patients receiving MV. Inoculation of the previously sterile lower respiratory tract results from aspiration of secretions, colonization of the aerodigestive tract, or use of contaminated equipment or medications [11].
The risk factors responsible for VAP occurrence can be classified into: host related, device related and personnel related. Host related factors include associated co-morbidities such as acute respiratory distress syndrome, cardiovascular system diseases, and central nervous system diseases; Multiple Organ System Failure (MOSF); level of consciousness; patients’ body positioning; number of intubations; and medications including sedatives and prior antibiotic administration [10]. Device-related factors include the endotracheal tube, the ventilation circuit and the presence of a nasogastric or an orogastric tube [12]. Personnel related factors include improper hand hygiene and inadequate use of personal protective equipment by the nursing staff, resulting in cross-contamination between patients [13].
The epidemiology and outcomes of VAP in adults were addressed in various studies [2,14,15]; However, fewer researches were conducted among paediatric patients [6,7]. To the researchers’ best evidence, no published data has been found regarding VAP in the PICUs at Cairo University Hospital.
Hence, this study aims to determine the incidence, risk factors and outcome of VAP in the PICUs at Cairo University Hospital egypt.
Materials and Methods
A hospital-based prospective cohort study was performed during the period from September 2014 to September 2015 to detect the incidence, causative organisms, risk factors, and outcomes of VAP. The study was conducted in two separate PICUs at the Paediatric Hospital, Cairo University; one at the emergency Department (included 24 beds and 24 ventilators) and the other at the 4th floor (included 14 beds and 14 ventilators).
All paediatric patients admitted to the selected PICUs for MV and fulfilling the inclusion criteria (n=427) were enrolled for the study. Inclusion criteria: Intubation and MV for at least 48h and patients older than one month-up to12 years.
Exclusion criteria: Neonates and pneumonia as admission diagnosis or detected within the first 48 hours. Patients were enrolled consecutively and were prospectively followed till VAP diagnosis, death or discharge from the PICUs, whichever came first. According to VAP occurrence, the study participants were divided into VAP and non-VAP groups.
A data collection sheet was used to collect and record the following: demographic data e.g., age and sex and clinical data including date of ICU admission; cause of admission (medical or surgical); associated co-morbidities; primary diagnosis (reason for initiation of ventilation); Paediatric Risk of Mortality (PRISM) III score; level of consciousness according to the Glasgow Coma Scale (GCS); duration of MV; length of ICU stay; occurrence of VAP; the causative organisms of VAP; duration of antibiotic use (<48h versus >48h before MV); positioning of patients (supine or semi-recumbent); reintubation; and the patients’ outcome (discharge, improvement or death). All cases were clinically examined (respiratory rate, color, use of accessory muscles, and auscultation to detect presence of rales) on admission and scored according to the GCS to detect the level of consciousness [16].
Cases were kept on broad spectrum antibiotic therapy before MV e.g. Tinam, Meropenam, Amikacin, Polymyxin, Ciprofloxacin and Vancomycin. Routine investigations were done for all patients e.g. Complete Blood Count (CBC), blood sugar, liver function tests, kidney function tests, blood culture, arterial blood gases and oxygen saturation. Baseline chest x-ray was done after intubation of patients on MV. It was repeated on follow up of patients when they developed signs of pneumonia. PRISM III score was assessed for all patients to detect the risk of mortality [17].
Symptoms and signs for patients under MV that raised suspicion for VAP included: altered mental state with no recognized cause, new onset of purulent secretions, change in sputum character, new onset or worsening of cough, increased respiratory secretions or suctioning requirements.
VAP was defined on the basis of clinical, radiologic and microbiological criteria. VAP was considered if it happened after 48 h of MV and was diagnosed based on the presence of a new persistent infiltrate on chest radiograph and at least two of the following: 1) fever of ≥ 38°C; 2) leucopenia (< 4000 white blood cells (WBC)/mm3) or leukocytosis (≥ 12,000 WBC/mm3); 3) purulent respiratory secretions; 4) worsening gas exchange (PaO2/FiO2 < 240) [18]. Early onset VAP: is that which occurred within 96 h of intubation and MV, while late onset VAP is that which occurred after 96 hour [10]. Microbiological workup was done once VAP was clinically suspected, endotracheal aspirates were performed; sputum was collected from patients from the tip of a suction catheter inserted in the endotracheal tube and transported in sterile tubes to the Clinical Pathology Department at Cairo University for diagnosis. Cultures were done using Blood, Chocolate and Mac-conkey agar systems, direct films were used for microscopic examination with gram stain to detect gram negative bacilli and gram positive cocci. VAP was established with a positive quantitative culture {cut-off point ≥ 106 (colony-forming units (CFU)/mL)} [19].
VAP incidence was calculated as follows: (Number of cases with VAP/Total number of patients who received MVx100) = VAP rate per 100 patients.
132/427x100= 30.9%
VAP incidence density was calculated as follows: (Number of cases with VAP/Number of ventilator days) x 1000= VAP rate per 1000 ventilator days [20].
132/6197 x 1000= 21.3 per 1000 ventilator days.
Sepsis: Defined as suspected infection in the presence of two or more Systematic Inflammatory Response Syndrome (SIRS) criteria [21]. SIRS refers to a clinical response to a non-specific insult, it entails 2 or more of the following: fever > 38°C or < 36°C, heart rate > 90 beats per minute, respiratory rate >20 breaths per minute, and abnormal WBC count (>12,000/mm3 or < 4000/mm3) [21]. MOSF: a progressive concurrent dysfunction of two or more organ systems; it is a non-specific expression of critical illness and a leading cause of death in critical care settings [21].
Approval of the study protocol was obtained from the Ethical Committee at the Faculty of Medicine, Cairo University. Informed consent was obtained directly from the legal guardian of each patient (mother, father, or other caregiver) before enrolment and after explaining the study objectives and importance. All administrative permissions were obtained from the Chairman of the PICUs where the study was conducted. All the procedures for data collection were treated with confidentiality according to Helsinki Declarations of biomedical ethics [22].
Statistical Analysis
Statistical analysis of the precoded data was done using the Statistical Package for Social Science Program (SPSS, version 21). The first part of the analysis examined the entire cohort of patients. Data were summarized using the median and Interquartile Range (IQR) for quantitative variables and frequency and percentage for qualitative variables. Univariate analysis was used for comparing variables for the outcome groups of interest (VAP versus no VAP), and all tests of significance were two tailed. The Mann-Whitney test was used for comparing quantitative variables, while the x2 statistic or Fisher-exact test was used for qualitative variables. The primary data analysis compared patients with VAP to those without VAP, and then the results of these tests were confirmed with a multivariate logistic regression analysis using a stepwise approach. The results of logistic regression analysis were reported as Adjusted Odds Ratios (AORs) with their 95% Confidence Intervals (CIs). Kaplan-Meier survival curve was done to demonstrate the predicted hospital survival in patients with and without VAP. All tests were considered statistically significant at a p-value of <0.05.
Results
A total of 427 paediatric patients meeting all the inclusion criteria were enrolled for the current study. Among these, VAP developed in 132 patients (30.9%). The median age of the studied group was 8 months (IQR= 5-36). The total ICU mortality was 54.6% [Table/Fig-1]. The most frequently encountered organisms from endotracheal aspirate cultures were Pseudomonas (47.7%), Acinetobacter (18.2%) and Methicillin-Resistant Staphylococcus aureus (MRSA) (14.4%). The incidence density of VAP was 21.3 cases per 1000 ventilator-days.
Characteristics of 427 mechanically ventilated patients at the paediatric intensive care units, Cairo University Hospital.
| Variable | Total (n=427) |
|---|
| Gender M/F n, (%) | 235/192, (55/45) |
| Age (months) median, (IQR) | 8 (5 - 36) |
| Cause of ICU admission medical/surgical n, (%) | 400/27, (93.7/6.3) |
| PRISM III on admission median, (IQR) | 10 (6 - 19) |
| Previous use of antibiotics > 48hrs n, (%) | 217 (50.8) |
| Primary diagnosis n, (%) | |
| Cardiovascular | 82 (19.2) |
| Neurologic | 166 (38.9) |
| Respiratory | 120 (28.1) |
| Sepsis | 31 (7.3) |
| Neuromuscular | 28 (6.6) |
| Complications of MVn, (%) | 167 (39.1) |
| VAP | 132 (30.9) |
| Pneumothorax | 41 (9.6) |
| Atelectasis | 18,(4.2) |
| Weaning n, (%) | 182 (42.6) |
| Reintubation n, (%) | 103 (24.1) |
| Supine position n, (%) | 306 (71.7) |
| Coma n, (%) | 116 (27.2) |
| MOSF n, (%) | 149 (34.9) |
| ICU Mortality n, (%) | 233 (54.6) |
| Duration of MV (days) median, (IQR) | 11 (5 - 17) |
| Length of ICU stay (days) median, (IQR) | 16 (8 - 22) |
Data are expressed as number and % for qualitative variables and median with interquartile range (IQR) for quantitative variables.
MOSF: Multi-Organ System Failure
Patients who developed VAP were significantly younger (median=6, IQR=4-18) than those who didn’t (median=12, IQR=5-42), p=0.013. A significantly higher percent of VAP patients presented with coma and MOSF compared to non-VAP ones (37.9% vs. 22.4%, p=0.001) and (60.6% vs. 23.4%, p<0.001), respectively. The VAP and non-VAP groups differed significantly regarding the primary diagnosis [Table/Fig-2].
Comparison between patients with and without ventilator-associated pneumonia regarding demographic and clinical risk factors.
| VAP | p-value |
|---|
| Yes (n=132) | No (n=295) | |
|---|
| Gender n, (%) |
| Male | 58 (43.9) | 177 (60) | 0.002* |
| Female | 74 (56.1) | 118 (40) | |
| Age (months) median, (IQR) | 6 (4-18) | 12 (5-42) | 0.013** |
| Coma n, (%) | 50 (37.9) | 66 (22.4) | 0.001* |
| MOSF n, (%) | 80 (60.6) | 69 (23.4) | <0.001* |
| PRISM III on admission median, (IQR) | 12 (6-24) | 10 (6-15) | 0.052** |
| Primary diagnosis n, (%) |
| CVS | 37 (28) | 45 (15.3) | 0.003* |
| CNS | 52 (39.4) | 114 (38.6) | 0.915* |
| Respiratory | 25 (18.9) | 95 (32.2) | 0.005* |
| Sepsis | 15 (11.4) | 16 (5.4) | 0.042* |
| Neuromuscular | 3 (2.3) | 25 (8.5) | 0.019* |
| Cause of ICU admission n, (%) |
| Medical | 121 (91.7) | 279 (94.6) | 0.284* |
| Surgical | 11 (8.3) | 16 (5.4) | |
Data are expressed as number and % for qualitative variables and median with (IQR) for quantitative variables.
*Chi square with Fisher exact
**Mann-Whitney test
A significantly higher percent of patients with VAP had antibiotics for more than 48 hour before MV (67.4% vs. 43.4%, p<0.001). Among VAP patients, 33.3% were re-intubated compared to 20% among the non-VAP ones, p=0.005. Regarding positioning of patients, 79.5% of the VAP group was in the supine position compared to 68.1% of the non-VAP group, p=0.02 [Table/Fig-3].
Comparison between patients with and without ventilator-associated pneumonia regarding treatment related risk factors.
| VAP | p-value |
|---|
| Yes (n=132) | No (n=295) |
|---|
| Previous use of antibiotics > 48 h n, (%) | 89 (67.4) | 128 (43.4) | <0.001 |
| Supine position n, (%) | 105 (79.5) | 201 (68.1) | 0.020 |
| Reintubation n, (%) | 44 (33.3) | 59 (20) | 0.005 |
| Weaning n, (%) | 44 (33.3) | 138 (46.8) | 0.011 |
Data are expressed as number and %, Chi square test of significance was used for comparison
The outcome of both study groups was shown in [Table/Fig-4]. The total duration of MV and that of ICU stay among patients with VAP (median=8, IQR=5-15 and median=11, IQR=7-18.5, respectively) was significantly shorter (p<0.001) compared to non-VAP patients (median=12, IQR=8-18 and median=17, IQR=10-23, respectively). Mortality rate among VAP patients was significantly higher compared to non-VAP patients (68.2% vs. 48.5%, P<0.001). [Table/Fig-5] shows that late onset VAP (>96 h) was significantly associated with higher mortality rate (77.6% vs. 58.5%, P=0.025) than early onset VAP (<96h).
Comparison between patients with and without ventilator-associated pneumonia regarding the outcome.
| VAP | p- value |
|---|
| Variable | Yes (n=132) | No (n=295) |
|---|
| Duration of MV median, (IQR) | 8 (5-15) | 12 (8-18) | <0.001** |
| LOS in ICU median, (IQR) | 11 (7-18.5) | 17 (10-23) | <0.001** |
| Mortality n, (%) | 90 (68.2) | 143 (48.5) | <0.001* |
Data are expressed as number and % for qualitative variables and median with (IQR) for quantitative variables.
*Chi square with Fisher exact
**Mann-Whitney test
Comparison between the onset of ventilator-associated pneumonia and mortality.
| VAP onset | |
|---|
| Mortality | Early (n=65) | Late (n=67) | p-value |
|---|
| N | % | N | % |
|---|
| Yes | 38 | 58.5 | 52 | 77.6 | 0.025 |
| No | 27 | 41.5 | 15 | 22.4 |
A backward stepwise logistic regression model was conducted to explore the significant predictors of VAP [Table/Fig-6]. Variables entered on 1st step were: gender, age in months, coma, MOSF, CVS disorders and sepsis (as an indication for MV), previous use of antibiotics > 48h, reintubation, and supine position. The last step revealed that only MOSF (OR=4.619, 95% CI 2.903-7.347, p<0.001), previous use of antibiotics > 48 h (OR=2.259, 95% CI 1.412-3.616, p=0.001), re-intubation (OR=1.794, 95% CI 1.076-2.991, p=0.025), coma (OR=1.733, 95% CI 1.051-2.856, p=0.031), and age in months (OR=0.990, 95% CI 0.983-0.998, p=0.013) were the significant predictors of VAP.
Logistic regression analysis of risk factors associated with ventilator-associated pneumonia among patients in the paediatric intensive care units.
| Variables | 95% CI | OR | p-value |
|---|
| MOSF | 2.903 - 7.347 | 4.619 | <0.001 |
| Previous use of antibiotics > 48hrs | 1.412 - 3.616 | 2.259 | 0.001 |
| Reintubation | 1.076 - 2.991 | 1.794 | 0.025 |
| Coma | 1.051 - 2.856 | 1.733 | 0.031 |
| Age in months | 0.983 - 0.998 | 0.990 | 0.013 |
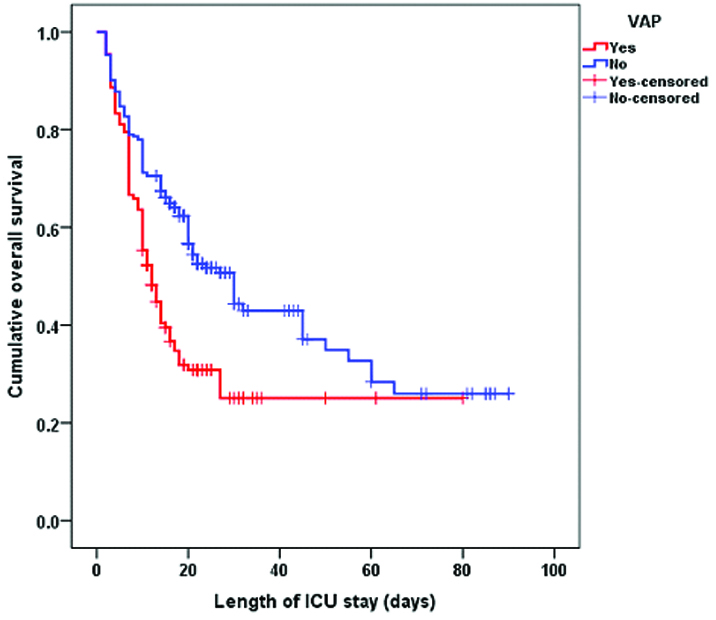
Kaplan-Meier survival analysis revealed that the median survival time for VAP patients (12, 95% CI 9.6-14.4) was significantly shorter (log rank, p<0.001) compared to non-VAP patients (30, 95% CI 23.7-36.3) [Table/Fig-7].
Kaplan-Meier survival analysis of patients relative to ventilator-associated pneumonia.
[Table/Fig-8] showed normal chest X ray for patient on MV in its 1st two hours then he developed by lateral lung infiltration on day three on MV.
Chest X Ray of patient on MV 1st hour and after 3 days.
Discussion
Although MV is an essential component of modern ICU care, it is associated with a considerable risk of VAP [23]. Proper recognition of high risk patients and of potential modifiable risk factors may outline preventive measures and institutional strategies to reduce the infection [2].
The incidence of VAP differs according to the studied population, type of ICU and the country income level [24,25]. In this study, the incidence of VAP was nearly 31%, which is relatively high compared to other figures in the literature. However, a lower figure was detected in another study conducted in Egypt to identify the rate of device-associated infections, where the incidence of VAP was 12.6% in the PICUs [9]. Thus, a considerable within-country variation can also exist which could be attributed to variations in VAP definitions, study methods, as well as the extent of application of infection control programs for preventing nosocomial infections. In a 30-month prospective study in a PICU in Saudi Arabia, the VAP incidence was 10.3% [26]. Moreover, Patria et al., estimated a VAP incidence of only 6.6% in the PICU at a University Hospital in Milan, Italy [27].
The incidence density of VAP in this study was 21.3 cases per 1000 ventilator days. In another study conducted in Egypt, the VAP rate in the PICUs was 31.8 per 1000 ventilator days [9]. In contrast, lower rates were reported in a 1-year prospective study in Australia, where the incidence density of VAP in a tertiary PICU was 7.02 per 1000 ventilation days [28]. Moreover, O’Brien et al., in the United States found a mean VAP rate of 1.33 per 1000 ventilator days [29].
The results of sputum cultures in the current study revealed that gram-negative bacteria were isolated from the majority of VAP patients (75.7%), with Pseudomonas aeruginosa and Acinetobacter predominance, while Methicillin-Resistant Staphylococcus aureus (MRSA) accounted for the majority of the gram-positive infections. Similarly, in a study conducted in Taiwan to determine the risk factors of VAP after paediatric cardiac surgery, Pseudomonas aeruginosa and MRSA were the most common pathogens isolated from the endotracheal aspirate [3]. In a study conducted by Aelami et al., publications addressing VAP in any inpatient paediatric population were analyzed, it was found that Pseudomonas aeruginosa, Acinetobacter and Enterobacteriaceae were the most common causative agents of VAP [30].
In this study, coma and MOSF were among the patient-related risk factors for development of VAP. Similarly, in a study conducted in the United States, patients with VAP had a greater incidence of coma (p=0.007) compared to those without [14]. Patients with VAP had a higher PRISM III score on admission compared to those without VAP, but no significant difference was observed (p=0.052). Roeleveld et al., found that VAP patients were more likely to have an admission PRISM III score of ≥ 10 (p=0.033) [31].
In this study, there was a significant association between the primary diagnosis of patients namely sepsis (p=0.042) and CVS disorders including congenital heart diseases (p=0.003) and the increased risk of VAP. However, in a study conducted in India, the primary diagnosis of patients enrolled (septicaemia, cardiac, neurologic and cancer) was similar in those who developed or did not develop VAP [32].
Treatment with antibiotics may affect the ecology of colonization by suppressing the normal flora and consequently increasing the risk of acquiring a nosocomial infection that is more likely to be drug-resistant [26]. Although prior antibiotic therapy has been recognized as a risk factor for VAP in previous studies of adults [33,34], it was rarely mentioned in studies of paediatric patients [26,35]. In this study, antibiotic use for >48h before MV was associated with a significantly higher risk of VAP (p<0.001). Similarly, Almuneef et al., found that prior antibiotic therapy was significantly associated with increased risk of VAP (p=0.005) [26]. In an effort to reduce the incidence of VAP in our PICU, rotation and appropriate use of antibiotics will be considered.
Many studies highlighted the role of aspiration in the pathogenesis of VAP [6,35]. Reintubation frequently implicates aspiration of gastric or oropharyngeal contents that are contaminated with colonizing flora. In the current study, reintubation was found to be a risk factor of VAP (p=0.005). Similar findings were reported by Srinivasan et al., Patria et al., and Chiru et al., [7,27,35]. In contrast Elward et al., and Gautam et al., did not find a higher VAP risk with reintubation [6,28].
Supine positioning may also facilitate aspiration leading to an increased risk of VAP [36]. In the present study, patients in the supine position had an increased risk of VAP compared to those in the semi-recumbent position. Measures to prevent aspiration in adults, including elevation of the head to 30°- 45°, continuous subglottic suctioning and continuous lateral rotation of the bed to improve drainage of lower airway secretions, are expected to be effective in children too [4,26]. Smulders et al., concluded that subglottic secretion drainage was associated with a relative risk reduction of VAP [37].
Several studies have showed that prolonged duration of MV and PICU stay were associated with increased VAP risk [3,32,35]. Surprisingly, in the current study, different findings were observed where the duration of MV and the LOS in PICU were significantly shorter in VAP patients compared to non-VAP ones. This finding could be attributed to decreased median survival time in the VAP group subjecting them to earlier mortality compared to the non-VAP one as shown by the Kaplan-Meier survival analysis. In contrast, Rello et al., stated that patients with and without VAP had similar in-hospital survival as shown by Kaplan-Meier curves, although the mortality rate was higher for patients without VAP during the first 30 hospital days [14].
In the present study, mortality rate in the VAP group was significantly higher compared to the non-VAP one. Similarly, patients with VAP had a higher mortality rate (p=0.04) in a study conducted in a PICU in Italy [27]. Ramya et al., and Alexis et al., also reported similar results [38,39]. In contrast, Huang et al., found that mortality rates of the VAP and non-VAP groups were similar in a PICU in Taiwan [40]. In the current study, distinction between early and late onset VAP was done in relation to the mortality rate, where patients with late onset VAP had a significantly higher mortality rate (P=0.025) than those with an early onset. However, differentiation between the two types is also important with regards to the causative organisms and thus the required treatment [14].
In the current study, significant variables on univariate analysis (p<0.05) were entered in a multivariate logistic regression model; only MOSF, prior antibiotic use >48h, reintubation, coma and age in months remained significant. In study conducted in the Netherlands, univariate analysis showed that risk factors for VAP in paediatric patients after cardiac surgery were: a PRISM score of ≥ 10 on admission, receiving fresh frozen plasma transfusions and a longer aortic cross-clamp time [31]. Only a PRISM score of ≥10 remained significant after a multiple logistic regression analysis. In another study conducted in Saudi Arabia, significant variables for VAP after multiple logistic regressions were only prior antibiotic therapy, continuous enteral feeding and bronchoscopy [26]. Chiru et al., found previous use of one or more antibiotics, antifungal drugs and reintubation to be positively associated with the development of VAP in children after a multivariate analysis [35].
Limitations
Among the study limitations, there was the lack of a gold standard for diagnosis, which may have limited the accuracy of the correlation with the risk factors and outcomes. Also, there was a difficulty in sampling procedures to obtain the microbiological specimens from the small respiratory tract of children. Invasive techniques to differentiate between infection and colonization such as Broncho Alveolar Lavage (BAL) could not be used as they were not practical and could be harmful in small infants.
Conclusion
The incidence of VAP in this study was relatively high which necessitates the need for possible improvements in the PICUs. The most prominent risk factors for VAP occurrence were MOSF, prior antibiotic use for >48h before MV, reintubation, coma and age. Late onset VAP was associated with poor prognosis. Awareness about the different risk factors will help in reducing VAP morbidity and mortality. Therefore, we recommend proper use of antibiotics before MV in PICUs to avoid the development of drug-resistant pathogens. Also, adequate training of nurses and strict supervision of infection control protocols are crucial. Measures to prevent aspiration resulting from reintubation are needed e.g. continuous subglottic suctioning. Further studies are warranted to evaluate the effect of these interventions in reducing VAP incidence in children.
Data are expressed as number and % for qualitative variables and median with interquartile range (IQR) for quantitative variables.
MOSF: Multi-Organ System Failure
Data are expressed as number and % for qualitative variables and median with (IQR) for quantitative variables.
*Chi square with Fisher exact
**Mann-Whitney test
Data are expressed as number and %, Chi square test of significance was used for comparison
Data are expressed as number and % for qualitative variables and median with (IQR) for quantitative variables.
*Chi square with Fisher exact
**Mann-Whitney test