Alarming increase in incidence of Diabetes Mellitus (DM) has been observed worldwide and is considered as one of the main threats to human health in the 21st century in both developed and developing nations [1]. Amongst the two types, Type 2 diabetes seems to be more prevalent illness. In adults, Type 2 diabetes accounts for about 90 to 95 percent of all diagnosed cases of diabetes [2]. Diabetic complications are the major cause of morbidity and mortality in persons with diabetes. The risk of developing diabetic complications depends on both the duration and the severity of hyperglycaemia [3]. Chronic hyperglycaemia is a major initiator of diabetic microvascular complications e.g., retinopathy, neuropathy, nephropathy [4]. Premature death in patients of diabetes has been reported, approximately in 50% due to cardiac complication and 10% by renal failure from the total of type 2 diabetics [5].
A dramatic increase in the prevalence of diabetic nephropathy has been noted in India as well, which has become the single most common cause of end-stage kidney disease [6]. The estimated overall incidence rate of Chronic Kidney Disease (CKD) in India is currently 800 per million populations [7].
Cataract, characterized by cloudiness or opacification of the eye lens, is the leading cause of blindness all over the world especially in developing countries [8]. One of the most important causes of visual impairment in subjects with Type 2 DM is diabetic maculopathy & cataract which causes blindness and visual morbidity [9].
In Modern medical a vast variety of measures are used like lifestyle modification and pharmacological interventions for preventing and controlling hyperglycaemia [10]. No remedies are available to prevent or treat these complications at early stage so patients tend to try agents from alternative systems of medicine. Herbal medicines promoted as effective and having no adverse effects. Nishamalaki (NA) is a combination of Curcuma longa and Emblica officinalis. Studies have demonstrated the antidiabetic effect of Nishamalaki [11,12]. Individual agents are found to be effective in preventing various complications of diabetes. Curcuma has been studied for renoprotective [13], cataract preventing [14] antioxidant, anti-inflammatory, antimicrobial, and anti-carcinogenic activities [15] and Embilica officinalis is rennoprotective, causes insulin release [16] hepatoprotective, gastroprotective, immunomodulator [17,18]. No studies are available on the Nishamalaki preparation for its protective effect on the prevention of diabetic complications.
Present study was aimed to study the protective potential of Nishamalaki in diabetic wistar rats on renal changes & delaying the progression of cataract.
Materials and Methods
IAEC approval (IAEC/BVDUMC/0139/2012-2013) was obtained and study was started. Wistar rats of either sex, weighing 150-200 gms were and 6-8 weeks used for the study. They were housed as per the CPCSEA guidelines in standard polypropylene cage 3 to 4 animals per cage. Animal coding was done according to standard protocol 12 hours day and night cycle was maintained food and water was provided ad libitum.
Diabetes induced with Streptozotocin (STZ) 60 mg/kg and 110 mg/kg Nicotinamide intra-peritoneally. STZ was dissolved in 0.1 M of sodium citrate buffer, with a pH of 4.5. It was kept in ice box during the use. Blood sugar levels (BSL) were measured 12 hourly. Rats showing BSL > 250 mg/dl after 48 hours were included in the study and animals were randomly allocated to different experimental groups.
Groups
Gr I: Control –Saline Treated
Gr II: DM Control- Saline Treated
Gr III: Nishamalaki Prophylactic (0.9 gm/kg)
(Nishamalaki started from day 0 after administration of STZ)
Gr IV: Nishamalaki Therapeutic (0.9 gm/kg)
(Nishamalaki given after development of Renal dysfunction)
Gr V: Enalapril (25 mg/kg)
Drug treatment of the prophylactic group started immediately after administration of STZ while remaining rats received no treatment till the development of renal dysfunction. After 8 weeks, renal dysfunction was confirmed with serum creatinine and BUN (Blood Urea Nitrogen).
Ayurvedic Formulation-consisting of Emblica officinalis (amla), Curcuma longa and honey was used for the study. Identification & Authentication of the rhizomes of curcuma longa and amla was done in Ayurvedic college of Bharati Vidyapeeth. Preparation was done as per the ayurvedic literature and standards compared with Ayurvedic pharmacopoeia of India [19]. All the animals were treated with the test drug and standard drug according to groups.
Drug treatment was given as per group for 30 days.
Eyes were examined for the developmental changes of Cataract. Grading was done for initiation, progression and maturation of lenticular opacity as follows [20].
Stage 0 – Clear lenses and no vacuoles present.
Stage 1 – Vacuoles cover approximately one-half of the surfaces of the anterior pole forming a sub capsular cataract.
Stage 2 – Some vacuoles have disappeared and the cortex exhibits a hazy opacity.
Stage 3 – A hazy cortex remains and dense nuclear opacity is present.
Stage 4 – A mature cataract is observed as a dense opacity in both cortex and nucleus [21].
Blood sample were collected-from retro-orbital plexus for biochemical analysis. Blood glucose was monitored initially for seven days after STZ injection and then once fortnightly to check for maintenance of hyperglycaemia. Parameters measured were - BSL, Creatinine & BUN. The duration required to development of diabetic complications was detected.
Rats were sacrificed on day 90 and kidney samples were collected. Histopathological analysis of kidney was done.
Statistical Analysis
Statistical Analysis was done with Graph pad Prism 6. ANOVA followed by Dunnett’s test was used for analysis. The p<0.05 was considered as significant.
Results
Blood glucose levels were elevated in all the groups after the injection of STZ except control. Rise in the BSL maintained throughout the study in diabetic control group. Prophylactic treatment significantly reduced (p<0.01) blood glucose in Group III compared to DM-Control. However, there was no significant change in BSL was seen in Enalapril treated group.
Serum Creatinine was also found to be increased significantly (p<0.01) in diabetic control group compared to control rats. Nishamalaki treatment significantly (p<0.01) reduced the elevated level of serum creatinine in diabetic rats from both therapeutic and prophylactic group. Though not significant, prophylactic treatment was more effective and showed the more promising results. [Table/Fig-1] shows the Creatinine & BUN levels in different groups of rats. Serum BUN levels were found to be increased significantly (p<0.001) in diabetic rats as compared to normal control rats. Treatment with Nishamalaki significantly (p<0.001) reduced the increased level of BUN in diabetic rats of Nishamalaki group when compared with diabetic control.
Biochemical analysis in blood
| Groups | Creatinine | BUN |
|---|
| I-Control | 0.66 ± 0.03 | 24.46 ± 1.40 |
| II-DM | 1.36 ± 0.08$$ | 69.11 ±4.3$$$ |
| III-Niasamalaki (Prophylactic) | 0.80 ± 0.02** | 26.57 ±1.01*** |
| IV-Nizhamalaki | 0.83 ± 0.02** | 29.35 ±1.01*** |
| V-Enalapril | 0.91 ± 0.03** | 31.26 ±1.02*** |
Comparison with control $$ p<0.01, $$$ p<0.001, Comparison with DM**p<0.001 & **p<0.001
Onset and Progression of Cataract: In control rats no cataract was developed, whereas in Diabetic control group cataract development started early and at the end of the study cataract was seen in all the rats [Table/Fig-2]. Two lenses were in stage 1&2, 7 lenses were in stage 3 and 3 lenses were in stage 4 from DM control group. In Prophylactic group 9 lenses were normal, 2 lenses were in stage 1and one lens was in stage 2. In Nishamalaki therapeutic group 5 lenses were normal, 4 lenses were in stage 1 and 3 lenses were with stage 2. In Enalpril group 2 lenses were normal, 5 lenses were in stage1,4 lenses were in stage 2, 1 lens was in stage3 [Table/Fig-3].
Rats from different groups showing development of Cataract.

Effect on progression of diabetic cataract.
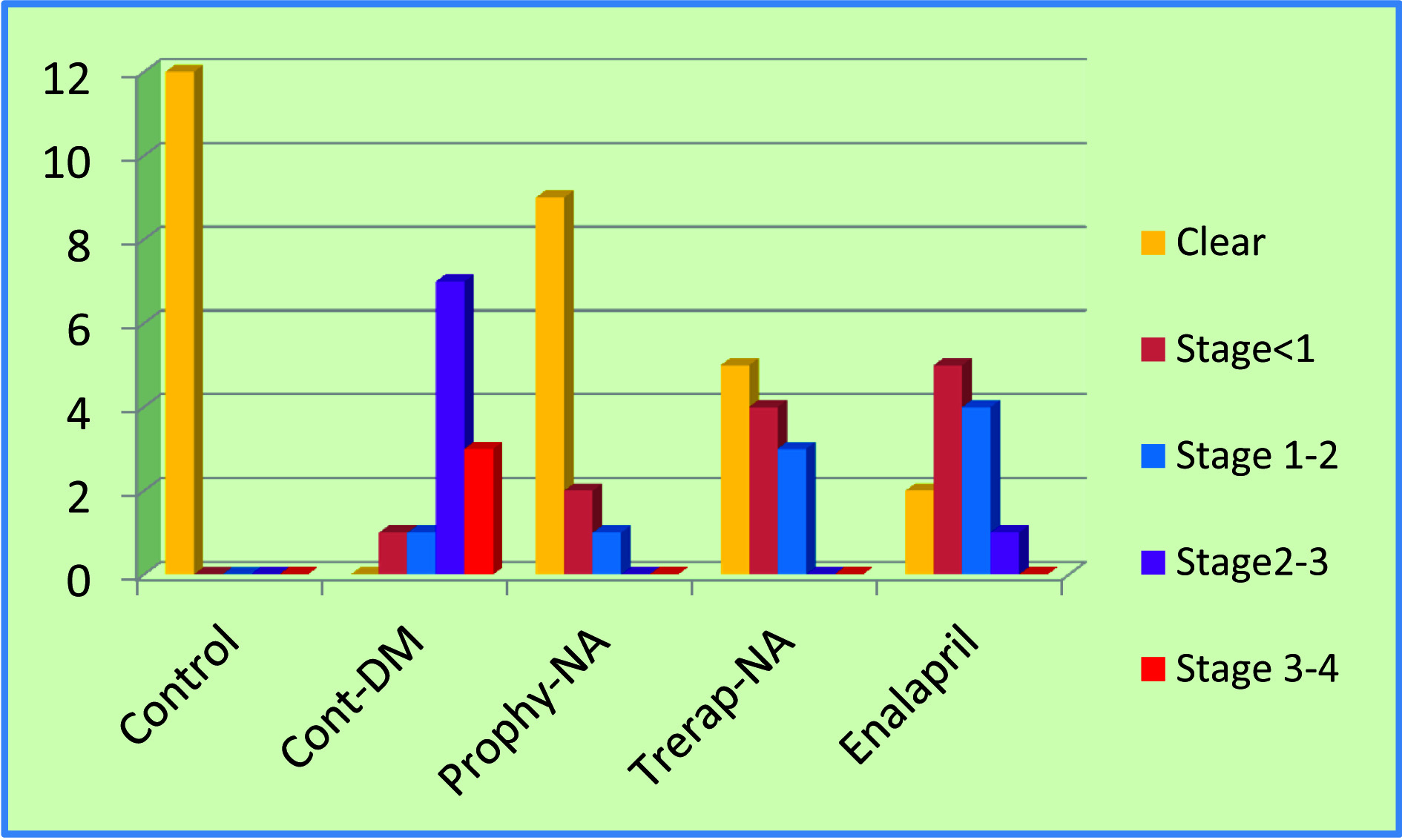
Group wise development of Cataract-
| Weeks | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Initiation | - | Gr II | - | - | - | Gr V | Gr IV | Gr III |
| Mature | - | - | - | Gr II | - | - | - | - |
Diabetic control -Gr. II: Cataract development started- 2th week, matured- end of 4th week.
Cataract development- Delayed in all treated groups, maximum in NA prophylactic (Gr III)- 8th week.
These results clearly indicate that treatment with Nishamalaki delayed progression of hyperglycaemia-induced cataract. Numbers of mature cataract lenses were significantly decreased in test groups in comparison with DM control group.
Histopathological Findings: The diabetic rats pathological changes were evident in the glomerulus with tubular injury as compared to normal rats. Pharmacological treatment with Nishamalaki prevented the diabetes-induced pathological changes in kidney. Rats on Nishamalaki showed near normal structures of renal tubules and no vacuolations.
Discussion
Prolonged hyperglycaemia leads to long term changes in stable macromolecules ending up in diabetic complications [22]. Cellular changes responsible for complications discovered so far are increased flux through polyol pathway, Production of advanced glycation end products, protein kinase activation and increased hexosamine pathway activity [Table/Fig-4] [23].
Consequences of hyperglycaemia.
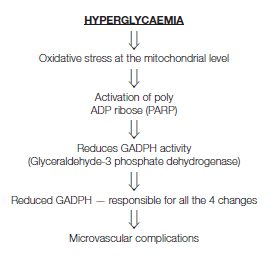
STZ is a pancreatic cell toxin that induces rapid and irreversible necrosis of cells [24]. STZ in the dose 60-250mg/kg, body weight has been demonstrated to induce complete destruction of cells in most species within 24 hour [25], Rapid development of diabetes which many times resembles to Type 1 diabetes mellitus. Addition of Nicotinamide helps to preserve some pancreatic cells and gives the appearance of type 2 diabetes which is a well known model of Type 2 diabetes.
Creatinine & BUN were reduced significantly in NA treated group, more marked effect was seen in prophylactic group showing protective action. These results are consistent with the study of Sharma et al., showing antioxidant action of curcumin responsible for the protective effect in-nephropathy [26] and the raised level of serum creatinine and urea nitrogen in the aged rats were decreased following the administration of E. officinalis extract and reduced age related renal dysfunction by oxidative stress in rats [27] Kasabari et al., showed E. officinalis insulin mimetic effect and also demonstrated inhibitory effects on α-amylase and α-glucosidase [28]. Curcuma has got protective role when used prophylactically and protects islets against streptozotocin-induced oxidative stress by scavenging free radicals effectively rescue islets from damage with no effect on normal cell function [29].
Nishamalaki delayed maturation of diabetic cataract due to slow progression. Although onset of cataract due to STZ-induced hyperglycaemia was not affected, progression and maturation were delayed significantly in a prophylactic and therapeutic Nishamalaki group. The protective effect was even more pronounced with prophylactic group. Our findings about the cataract development in Nishamalaki are consistent with the Suryanarayana et al., that turmeric delays STZ-induced cataract [30,31].
Histopathology of kidney showed tubular cell necrosis, tubular lumen dilation, foci of denuded basement membrane, vacuolization, pyknotic nuclei in diabetic group. With NA & Enalapril, near normal glomerular and tubular structures compared with the shrunken glomerulus, tubular vacuolations in DM control group [Table/Fig-5a-e].
(a) Control group-Control group kidney showing normal structure of glomerulus (H & E 10X). (b) Diabetic control group-Glomerulo-sclerosis, tubular lesions and cellular infiltration. (c) Nishamalaki Prophylactic-showing reduced renal pathology. (d) Nishamalaki Therapeutic-showing reduced renal pathology. (e) Enalapril-showing reduced renal pathology.

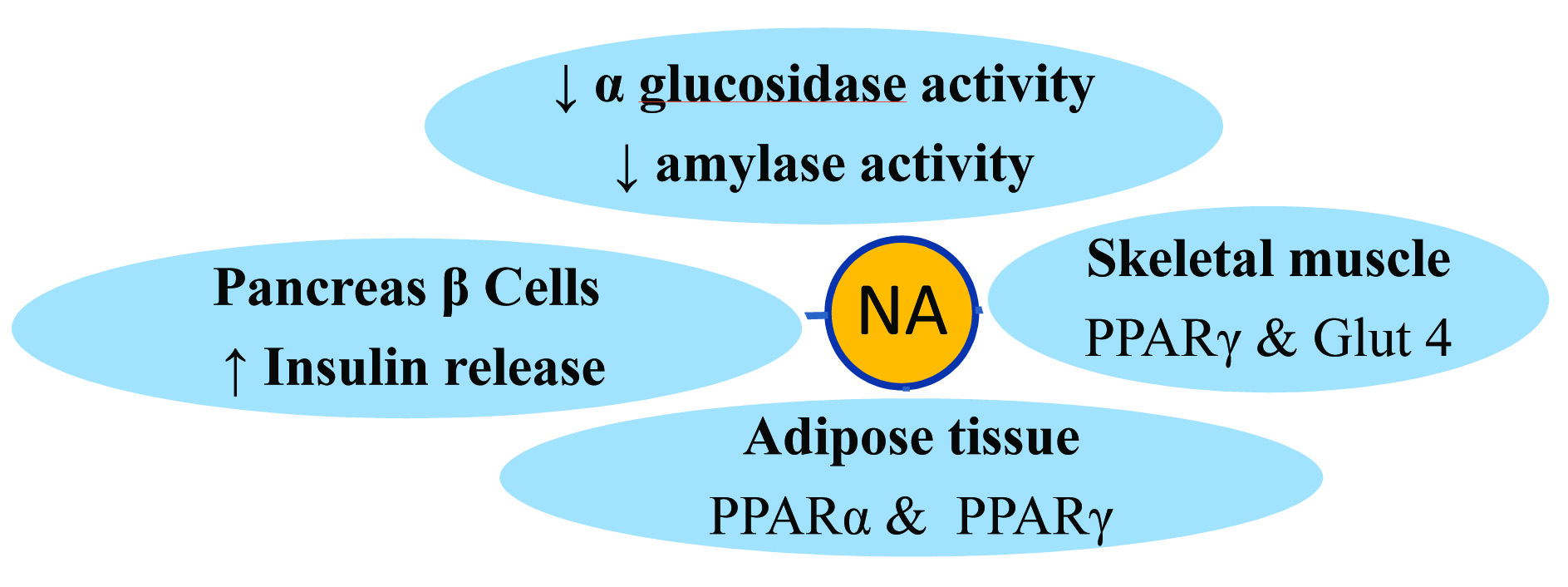
Protective effect may be seen because of the synergistic combination of Curcuma Longa and Emblica officinalis contributing through [Table/Fig-6] possible different mechanisms like- increasing Insulin sensitivity, increasing Glucose Uptake, decreasing α glucosidase activity, decreasing Amylase activity, increasing Insulin release, decreasing Oxidative stress. Nishamalaki through multiple actions has showed protective effect against diabetic nephropathy and also prevented and delayed development of cataract. Thus, Nishamalaki appeared to be more useful in the prevention and treatment of diabetic complications.
Nishamalaki – Synergistic effect of combination.
Conclusion
Once the morphological alterations in diabetic nephropathy sets in, they are not reversible leading to end stage renal disease and same is true for the cataract leading to blindness. But better control of hyperglycaemia and reduction in oxidative stress delays the progression of complications. Nishamalaki showed protective effect on diabetic nephropathy & also delayed the progression of cataract in rats. Nishamalaki administration observed to be more effective in prophylactic groups. Dietary use of curcuma longa and Emblica officinalis show beneficial effects but the scientifically prepared formulations is definitely effective as alternative treatment strategies for prevention of diabetic nephropathy and development of cataract. In present study biochemical findings were supplemented by the histopathology observations. In the dose of 60 mg/kg streptozotocin, very rapid development of diabetes with high mortality was the limitation of study. For the assessment of exact duration of prophylactic use of drug administration, further studies are required with the animal model mimicking the diabetes development in the human i.e. low dose STZ and high fat high fructose.
Comparison with control $$ p<0.01, $$$ p<0.001, Comparison with DM**p<0.001 & **p<0.001