Wine Glass Sign and Empty Delta Sign: A Rare Imaging Presentation of Postpartum Encephalopathy in Dehydration
Chanabasappa V. Chavadi1, K. Suprasanna2, Anees Dudekula3, Madhav Hegde4, Swetha Kory5
1 Assistant Professor, Department of Radiology, Kasturba Medical College, Manipal University, Mangalore, Karnataka, India.
2 Assistant Professor, Department of Radiology, Kasturba Medical College, Manipal University, Mangalore, Karnataka, India.
3 Resident, Department of Radiology, Kasturba Medical College, Manipal University, Mangalore, Karnataka, India.
4 Associate Professor, Department Radiology, Dr. B.R. Ambedkar Medical College, Bengaluru, Karnataka, India.
5 Resident, Department of Pathology, Yenepoya Medical College, Mangalore, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Chanabasappa V Chavadi, Assistant Professor, Department of Radiology, Kasturba Medical College, Manipal University, Mangalore-575001, Karnataka, India.
E-mail: chavadidoc@gmail.com
Rapid correction of hyponatremia is a well-known cause of central pontine and extrapontine myelinolysis. But uncommonly seen and rarely reported in Hypernatraemia. We report a rare case presenting as postpartum psychosis, wherein imaging revealed myelinolysis of corticospinal tracts in wine glass distribution and empty delta sign due to cortical venous thrombosis. At follow-up 3 months later, revealed significant neurological improvement. Concurrance occurrence of this dual pathology is not been described, which in our case was due to high serum sodium levels at presentation and dehydration.
Hypernatraemia, Magnetic resonance imaging, Myelinolysis, Thrombosis
Case Report
A 29-year-old female presented to the Emergency Department with history of altered sensorium, irrelevant talking and fever since 3 days, left hemiparesis since one day and generalized tonic clonic seizures on the day of admission. She had delivered a full term baby 17 days back. There was no significant antenatal or perinatal history. The patient was not a known hypertensive or diabetic.
On general physical examination the pulse rate was 110/min, blood pressure 110/70 mm of Hg, respiratory rate 24/min and body temperature of 1000 F. On examination she had altered higher mental functions, left upper limb power of 0/5, left lower limb power of 2/5 and right side limbs power was normal. Examination of other systems was unremarkable. Biochemical investigations were as follows: Sodium-164 mEq/L, Potassium-3.5 mEq/L, total leucocyte count-8200 cells/μL, Hb-9 gm/dl, Platelet-2 lac/cu mm, PT/INR-normal. No electrolyte correction was done prior to MRI examination.
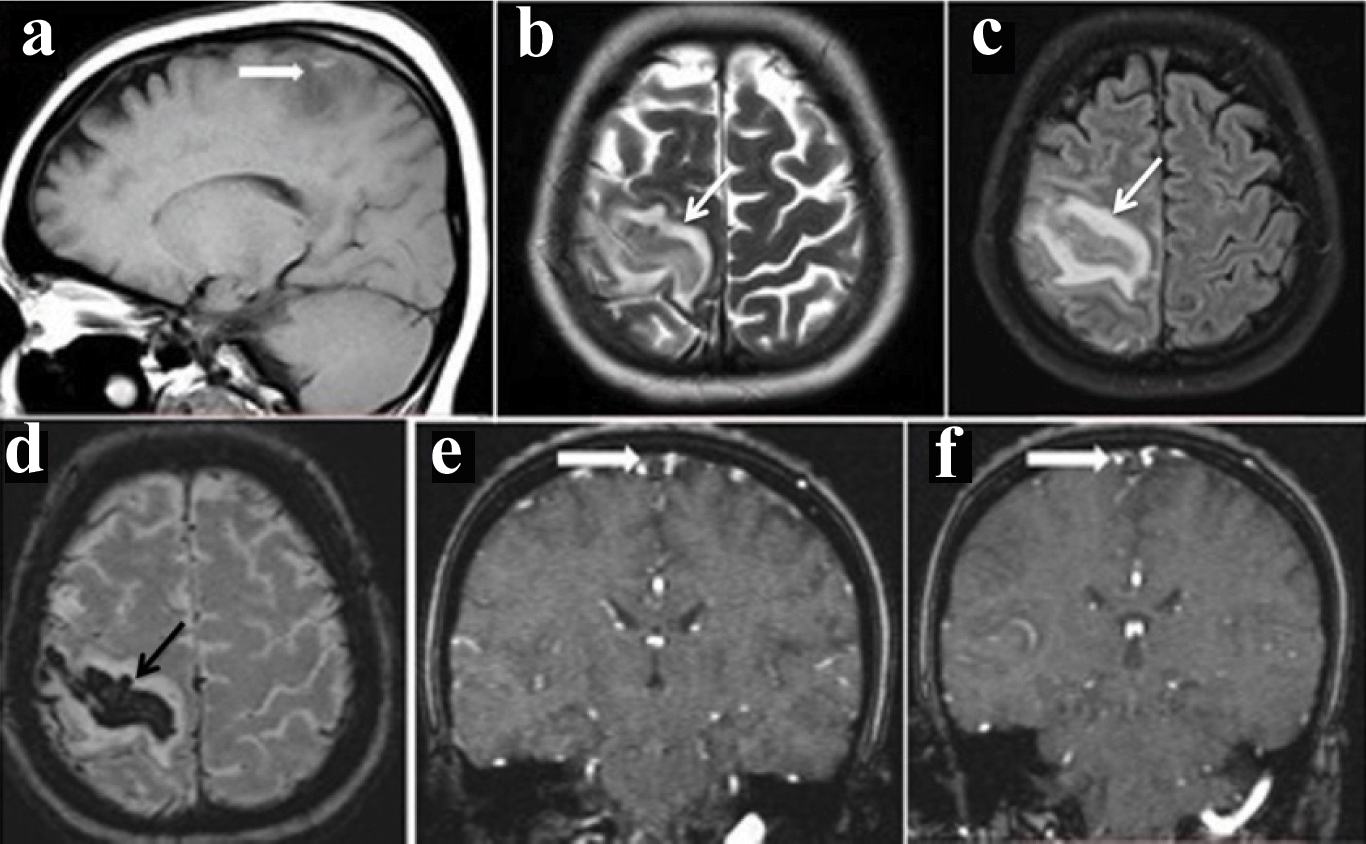
Magnetic Resonance Imaging (MRI) revealed altered signal intensity in the region of right perirolandic gyrus with surrounding oedema [Table/Fig-1a-c], corresponding blooming on Susceptibility weighted Imaging (SWI) [Table/Fig-1d], 2D TOF MR venogram demonstrated absence of flow in superior sagittal sinus [Table/Fig-1e&f].
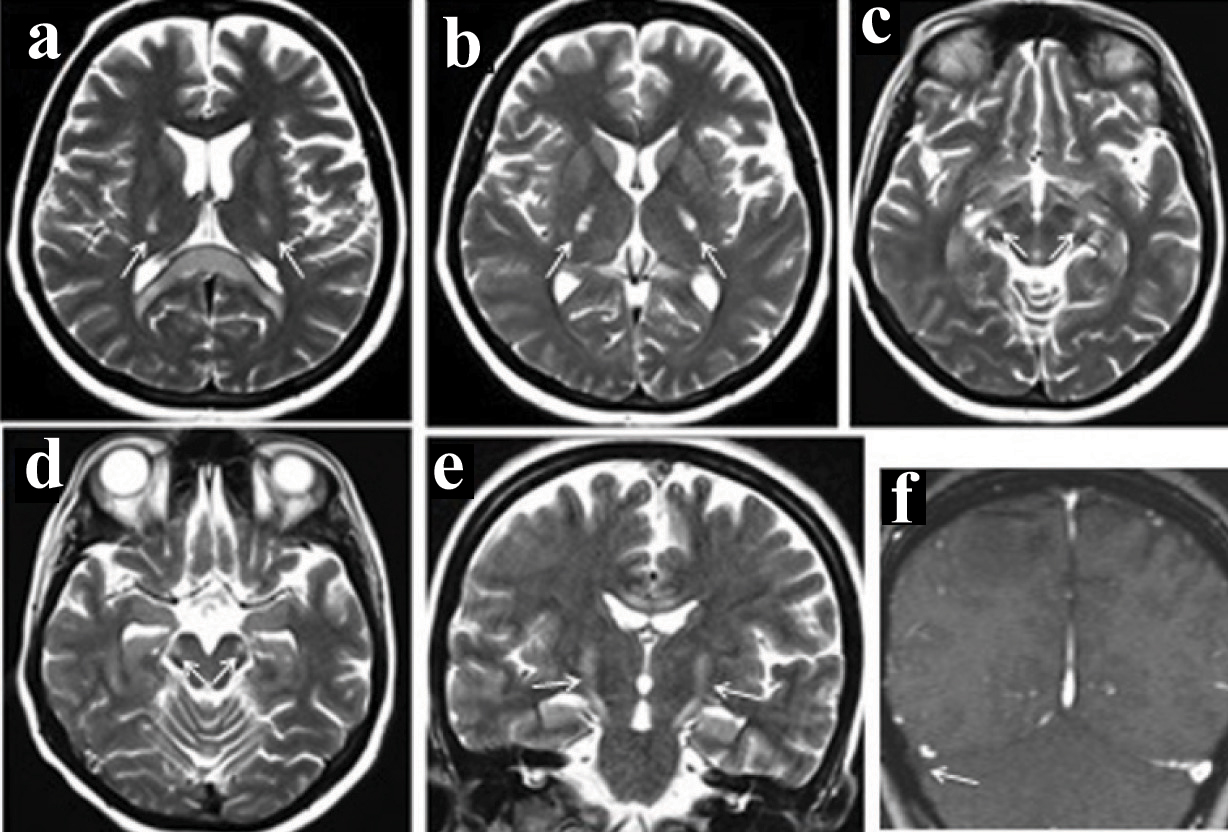
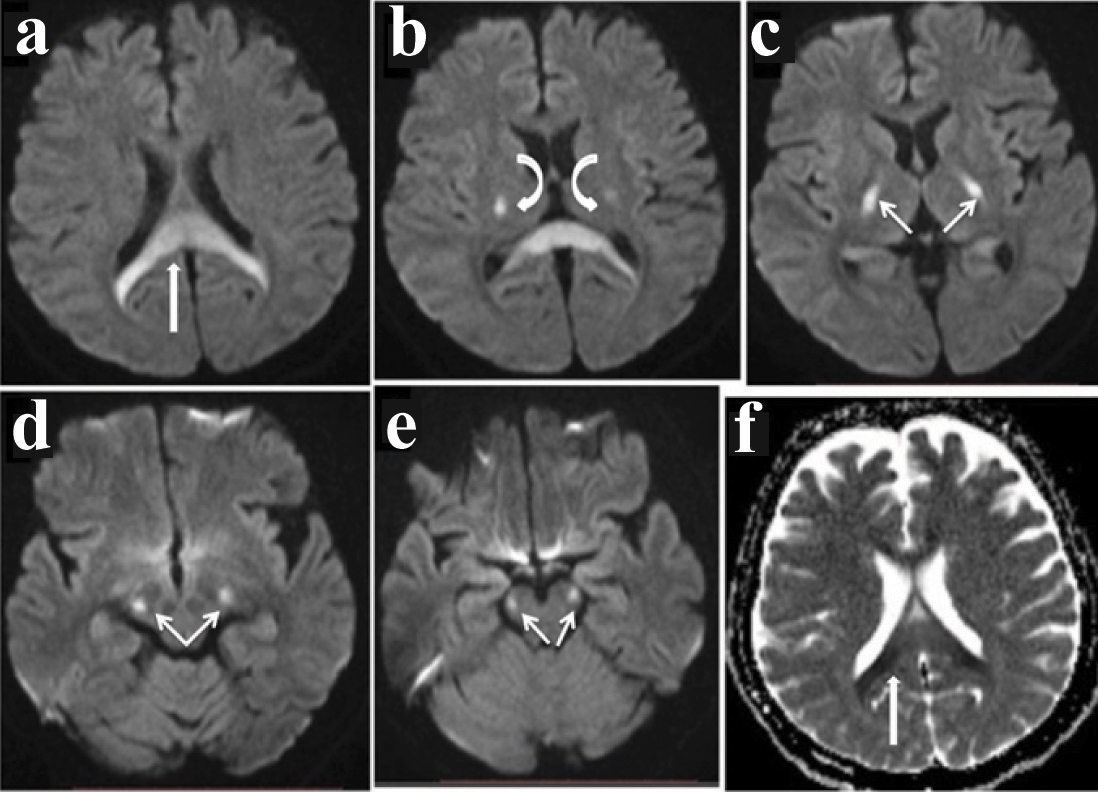
MRI revealed bilateral symmetrical non enhancing T1 hypointense, T2/Fluid Attenuation Inversion Recovery (FLAIR) hyperintense area showing diffusion restriction in corona radiata, splenium of corpus callosum, posterior limbs of internal capsule, ventrolateral thalami, lateral aspect of cerebral peduncles, anterolateral midbrain and cerebellar peduncles [Table/Fig-2,3a-e]. The involvement of corticospinal tract was also noted on coronal images [Table/Fig-2e] in Wine glass pattern. MR venogram showed absence of flow in the right sigmoid sinus [Table/Fig-2f]. ADC image [Table/Fig-3f] showed corresponding decreased signal in the splenium of corpus callosum.
(a)- Sagittal T1 image show hyperintensity with adjacent oedema; (b&c)- correspondingaxial regions in T2 and FLAIR show hypointensity and adjacent oedema; d- Axial susceptibility weighted(SWI) show blooming suggestive of haemorrhage; (e&f)- Coronal time of flight (TOF) venogram show absence of flow in the superior sagittal sinus (empty delta sign) indicating thrombosis.
Bilateral symmetrical hyperintensity in ventrolateral thalami & splenium of corpuscallosum in image (a), Posterior limb of internal caspule in image (b), Mid brain in image (c&d); Coronal T2 image show “Wine Glass” appearing hyperintensity involving the corticispital tract (e). Right transverse sinus thrombosis on MR venography (f).
Diffusion weighted images show hyperintensity in splenium of corpus callosum in image (a), bilateral symmetrical hyperintensity in ventrolateral thalami in (b), Posterior limb of internal capsule in image (c), Mid brain in image (d&e); ADC image in (f) show corresponding decreased signal in the splenium of corpus callosum.
These MRI signal changes confirmed the presence of a haemorrhagic infarct in the right perirolandic gyrus, presence of a venous thrombosis in mid superior sagittal thrombus and right sigmoid thrombus with coexisting hypernatraemic myelinolysis involving corticospinal tract and corpus callosum. The patient recovered with correction of hypernatraemia and heparin anticoagulation. At follow-up 3 months later, revealed significant neurological improvement.
Discussion
Similar cases of hypernatremia myelinolysis have been reported in literature, which were treated with parenteral fluids and steroids [1,2]. Rapid correction of hyponatremia is a well-known cause of central pontine and extrapontinemyelinolysis. The osmotic endothelial injury results in formation of toxins which causes myelinolysis and oedema as confirmed by post-mortem examination of tissue by histology. An inflammatory reaction is conspicuously absent differentiating it from pontine infarction and inflammatory demyelinating diseases. These patients present with varying degrees of encephalopathy, seizures, rhabdomyolysis, and rarely subdural haematoma [3]. Hyponatraemic myelinolysis occurs only after rapid electrolyte correction however myelinolysis in hypernatraemia is encountered both following rapid correction as well as primarily with elevated sodium levels [4]. The anatomic involvement in hyponatraemic myelinolysis can be either central pontine and/or extrapontine regions.
Indexed patient had dehydration at presentation with dry tongue, history of reduced oral intake of fluids due to altered mental status, with subsequent haemoconcentration probably precipitated the cerebral venous thrombosis. Central adypsic hypernatraemia with hypothalamic dysfunction can result in absence of thirst sensation and dehydration. Partial ADH secretion defect may manifest in pregnancy due to the enhanced peripheral breakdown by vasopressinase [5]. Hypernatraemic myelinolysis has been described in postpartum period and when associated with fever and poor oral fluid intake precipitates dehydration leading to increased sodium levels in the blood. It is seen that steady rise in serum sodium levels is enough to precipitate osmotic demyelination and rapid correction of hypernatraemia is not necessary in all cases, as seen in indexed patient. This pattern of involvement was not seen in hyponatraemic demyelination and moreover the pattern of involvement is different in both the scenarios. There has been no previous citation of concurrent occurrence of cerebral venous thrombosis and hypernatraemicmyelinolysis in literature.
The “Mexican hat sign” is what is typically described in hyponatraemicmyelinolysis and a “Wine glass sign” is described in hypernatraemicmyelinolysis, which was demonstrated in indexed patient [6]. Mexican hat sign is seen in the pons which is the area typically affected in hyponatraemic central pontine myelinolysis. Wine glass sign consists of symmetrical bilateral white matter hyperintensities on coronally obtained T2 images in corona radiata, posterior limbs of internal capsule, thalamus, cerebral peduncles, anterolateral midbrain and pons [7] [Table/Fig-2e]. Wine glass sign is seen only in advanced cases where there is involvement of the corticospinal tracts.
The main differential diagnosis of hypernatraemic myelinolysis is metronidazole toxicity where signal intensity changes are noted in cerebellar dentate nuclei, midbrain, dorsal pons, splenium of the corpus callosum, and the dorsal medulla. However, the obvious clinical history of chronic diarrhea and metronidazole usage will be present [8]. Another differential can be amyotropic lateral sclerosis where symmetrical hyperintensities of the internal capsule, crus cerebri, and pons similar to the ’wine-glass’ pattern’ can be seen however corpus callosal involvement is not seen [9].
Conclusion
Since myelinolysis is usually encountered after rapid correction of hyponatremia. Hypernatraemia is a less recognized and relatively rare cause of myelinolysis. Here we report a case of hypernatraemic myelinolysis to aquaint the reader with its typical radiological findings. Moreover, the simultaneous occurrence of hypernatraemic myelinolysis and cerebral venous thrombosis in the same patient has not been described previously in literature, what makes this case report unique.
[1]. Bhatia S, Kapoor AK, Sharma A, Gupta R, Kataria S, Cerebral encephalopathy with extrapontinemyelinolysis in a case of postpartum HypernatraemiaIndian J Radiol Imaging 2014 24(1):57-60. [Google Scholar]
[2]. Han MJ, Kim DH, Kim YH, Yang IM, Park JH, A case of osmotic demyelination presenting with severe HypernatraemiaElectrolyte Blood Press 2015 3(1):30-36. [Google Scholar]
[3]. Adrogue HJ, Madias NE, HypernatraemiaN Engl J Med 2000 342:1493-99. [Google Scholar]
[4]. Yoon SH, Park J-Y, Choi S-U, Yoon SZ, Lee HW, Central pontine myelinolysis in a patient with persistent mild Hypernatraemia following cadaver donor liver transplantationKorean J Anesthesiol 2013 65(1):87-88. [Google Scholar]
[5]. Shrier RW, Systemic arterial vasodilation, vasopressin, and vasopressinase in pregnancyJ Am SocNephrol 2010 21:570-72. [Google Scholar]
[6]. Islam O, Dhillon G, MRI in Osmotic Demyelination: The “Mexican Hat” SignThe Internet Journal of Radiology 2009 12:1 [Google Scholar]
[7]. Saroja AO, Naik KR, Mali RV, Kunam SR, ’Wine Glass’ sign in recurrent postpartum hypernatraemic osmotic cerebral demyelinationAnn Indian Acad Neurol 2013 16:106-10. [Google Scholar]
[8]. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH, Imaging of metronidazole induced encephalopathy: Lesion distribution and diffusion-weighted imaging findingsAm J Neuroradiol 2007 28:1652-58. [Google Scholar]
[9]. Agosta F, Chio A, Cosottini M, De Stefano N, Falini A, Mascalchi M, The present and the future of neuroimaging in amyotrophic lateral sclerosisAm J Neuroradiol 2010 31:1769-77. [Google Scholar]