Introduction
Thoracic endometriosis syndrome is a rare disorder characterised by the presence of functioning endometrial tissue in pleura, lung parenchyma, airways, and/or encompasses mainly of four clinical entities–catamenial pneumothorax, catamenial haemothorax, catamenial haemoptysis, lung nodules [1]. Of these, catamenial pneumothorax is by far the most common clinical presentation [2].
Catamenial pneumothorax is a condition of collapsed lung (pneumothorax) occuring in conjuction with menstruation (catamenial refers to menstruation), believed to be caused primarily by endometriosis of the pleura [3].
The diagnosis of TES has improved over the past two decades because of video assisted thoracoscopic surgery and laparoscopy coupled with a higher level of clinical suspicion. The aetiology and pathogenesis however, are still not well understood. Management is by combined approaches being reported in the medical literature [1].
Clinical Presentation
Case 1
A 40-year-old woman, P1L1, presented in April 2012 with catamenial breathlessness of two years duration, with past history of bilateral pleural effusion in 2011, following which 1.5L of haemorrhagic fluid was drained at a nearby hospital. She was started empirically on Anti Tubercular Treatment which was discontinued after 2 months due to drug induced hepatitis. She also had a past history of myomectomy done in 2009.
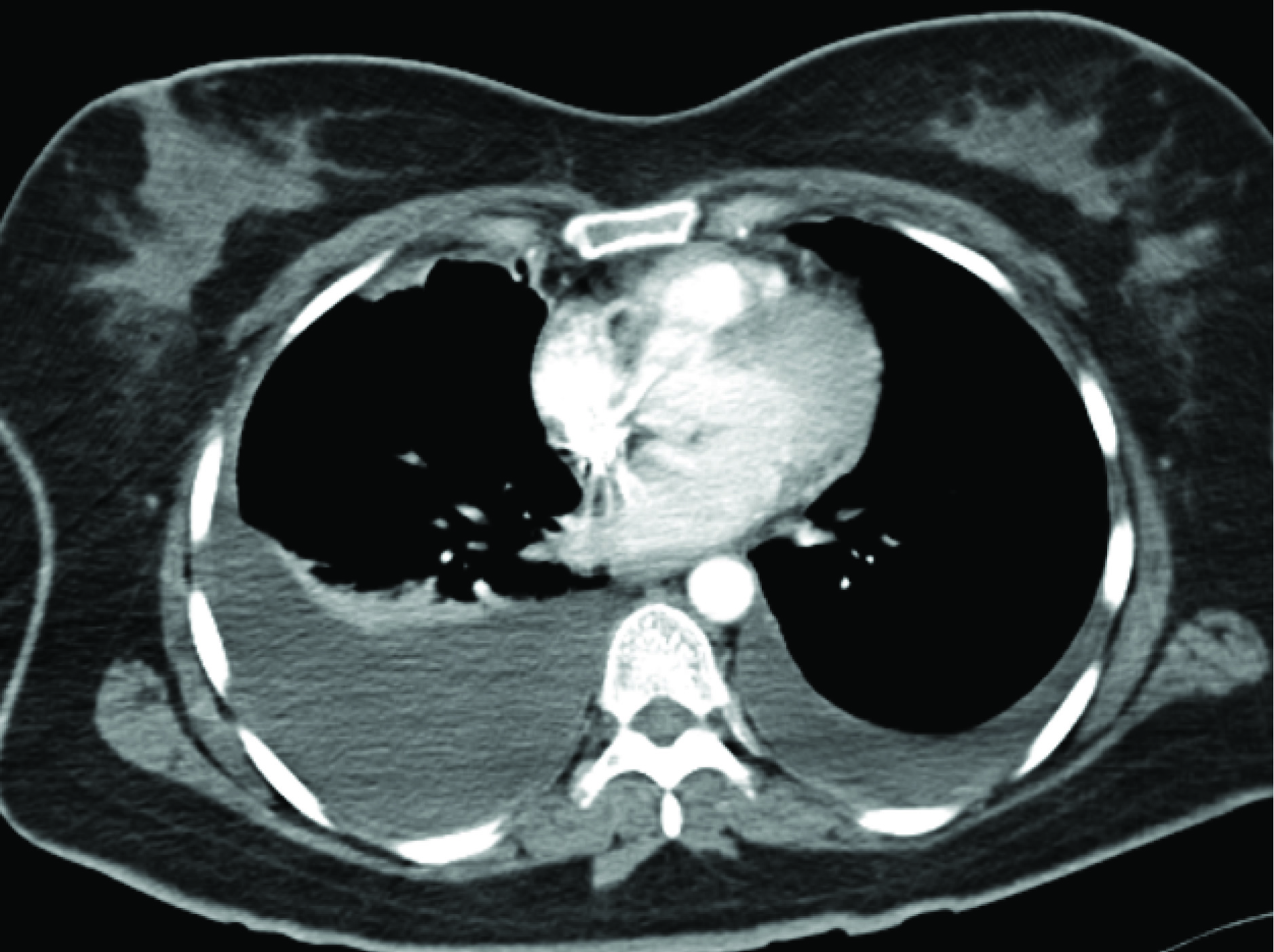
On evaluation her Chest X ray (CXR) and CT scan there was evidence of bilateral pleural effusion more on the right side than the left [Table/Fig-1]. One litre of haemorrhagic fluid was drained. Pleural fluid analysis was haemorrhage with degenerated RBC. Her ultra sound showed adenomyosis and CA 125 was 405.7 IU/l. She was worked up and diagnosed as TES. Thoracoscopy was advised but patient refused. Hence she was given INJ DMPA 150mg IM of (6 doses).
CT Scan–Bilateral Pleural Effusion.
In 2012 August, she again developed catamenial breathlessness. Symptomatic treatment was given followed by hysterectomy with bilateral salpingo ophorectomy. In 2013 May, she had breathlessness, cough, and chest discomfort of one week duration. Thoracoscopy was done and pleural nodules were seen. Pleural biopsy showed endometriotic nodules. Inj. GnRH was given for 12 months along with calcium supplements. Post treatment she is asymptomatic for more than a year.
Case 2
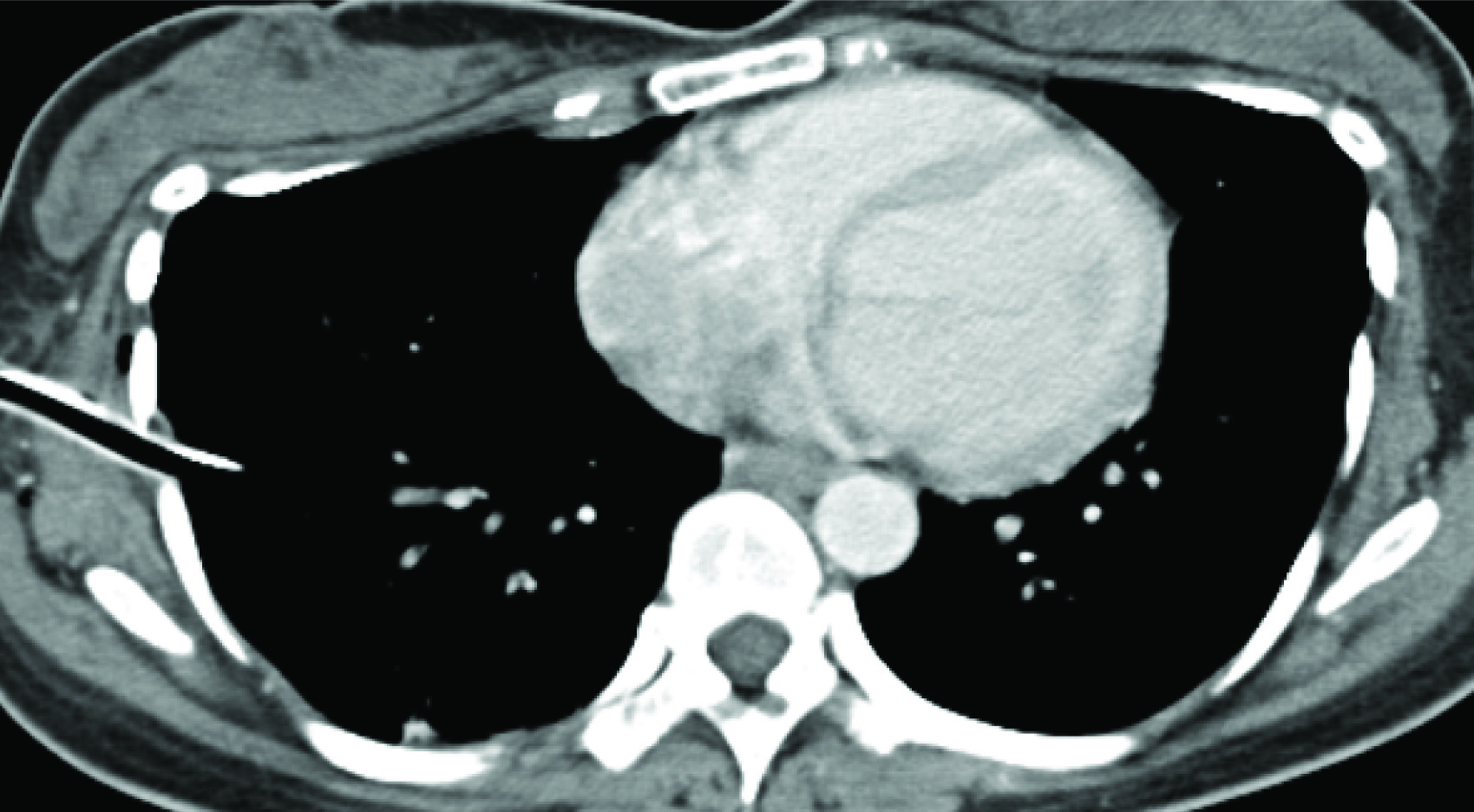
A 36-year-old female presented in June 2012, P2L2, marfanoid habitus, had catamenial breathlessness with right sided chest pain of 4days duration. Her CXR and CT scan showed right pnuemothorax [Table/Fig-2]. Intercoastal drainage [Table/Fig-3] was performed and right pleurodesis with betadine was done. She had a recurrence in December 2012 which was mild and was resolved with conservative management. Her pelvic scan showed uterine fibroid. Till now she is asymptomatic (2years).
CT Scan–Right Pneumothorax with ICD Tube.
Case 3
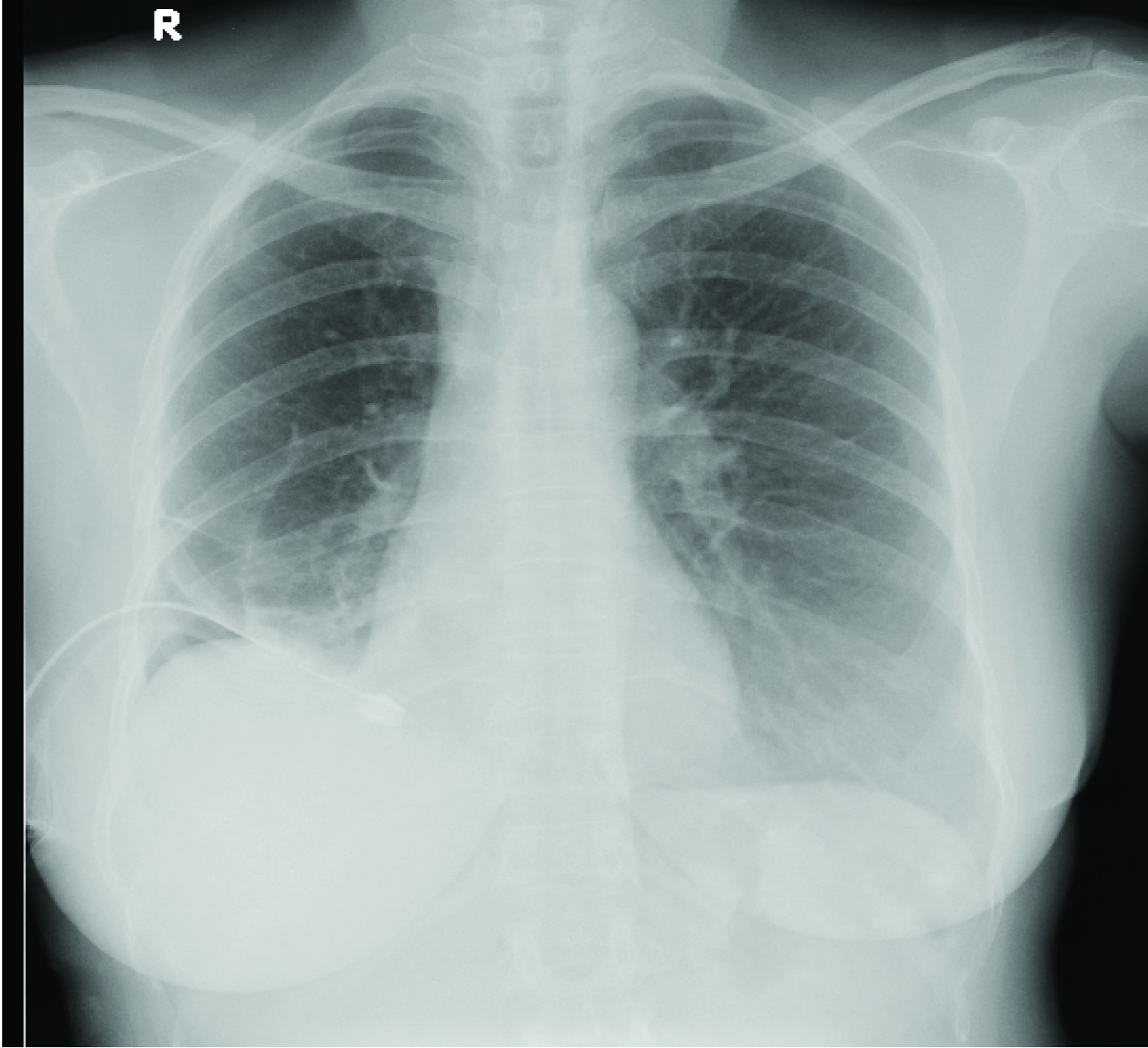
A 43-year-old nulligravida presented in October 2013 with catamenial breathlessness and non-productive cough of 4 months duration. CT scan showed pneumothorax on right side for which tube thoracostomy was done [Table/Fig-4]. Again in February 2014; she had recurrence of catamenial breathlessness and CXR showed pnuemothorax [Table/Fig-5]. Thoracoscopy was done and was found to be normal. Pleurodesis with betadine was perfomed. Pleural biopsy showed histiocytes and fibrofatty tissue. She was planned for pleurectomy with hysterectomy and bilateral salpingo ophorectomy on the next recurrence. But till now she is asymptomatic.
CT Scan–Right Sided Pneumothorax with Collapse of Posterior Basal Segment on Right Side (Drainage Tube Insitu).

CXR–Lung Colapse with Right Pneumothorax with ICD Tube.
Case 4
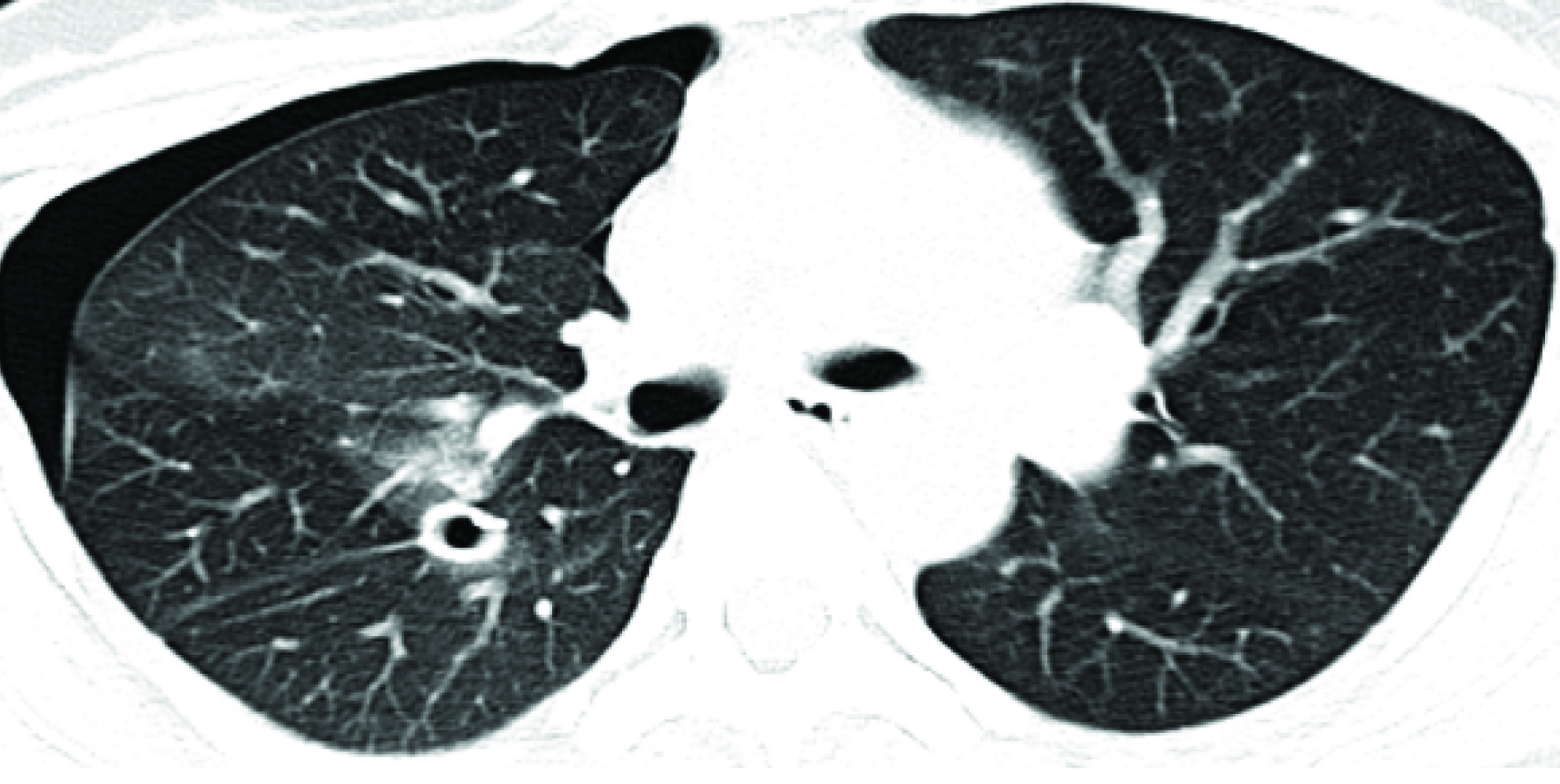
A 34-year-old female presented in December 2013, P2L2, marfanoid habitus, developed right upper backache, catamenial chest discomfort of 3days duration. Her CXR showed right pneumothorax. CT scan report also showed right pneumothorax with plaque like lesion on the pleural base suggestive of endometriosis [Table/Fig-6]. Her ultrasound pelvis showed focal adenomyosis. She was given supportive treatment in November 2014. She had mild recurrence of pneumothorax for which conservative management was done.
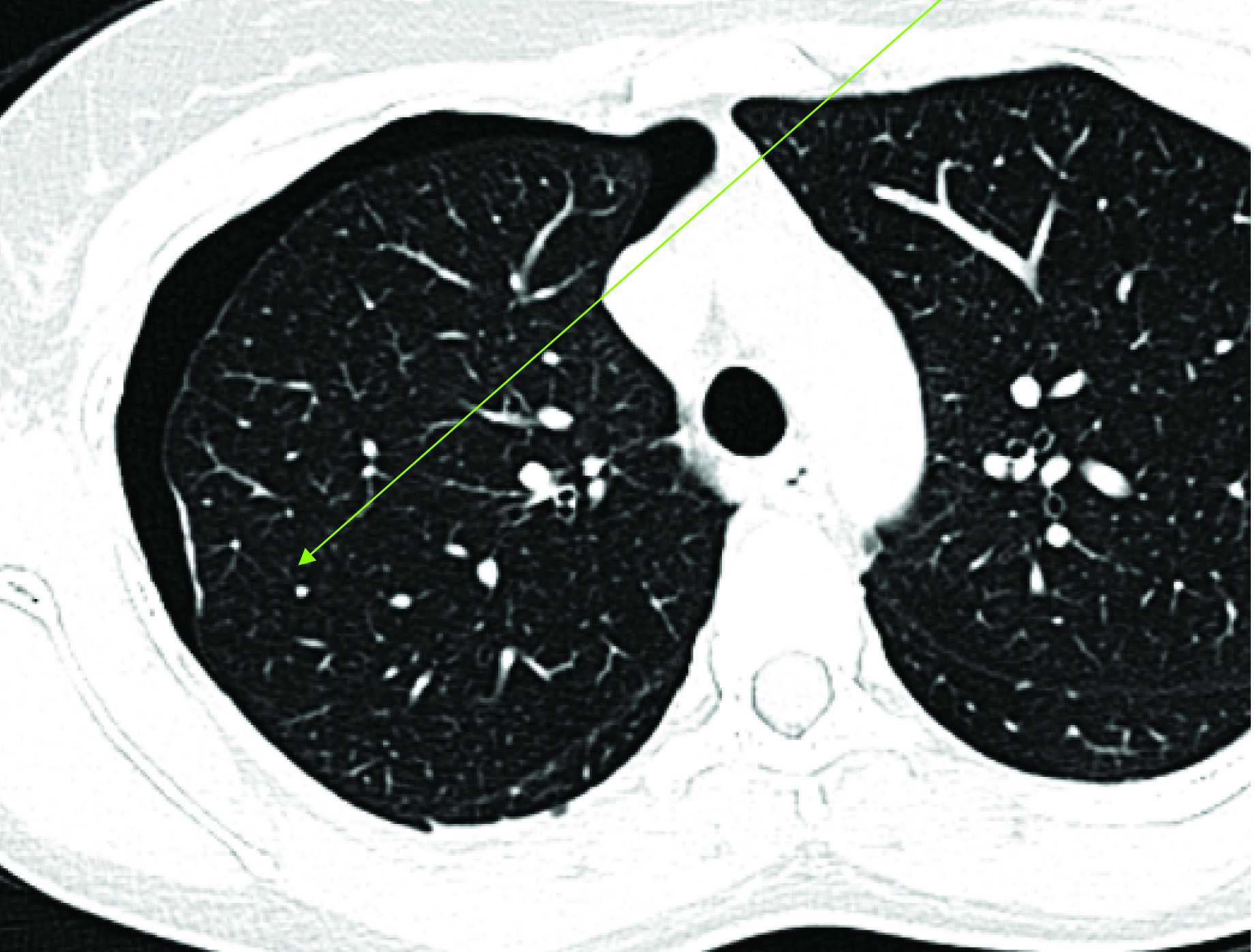
CT Scan–Pneumothorax with Pleural Endometriosis (Plaque Like Lesion).
Case 5
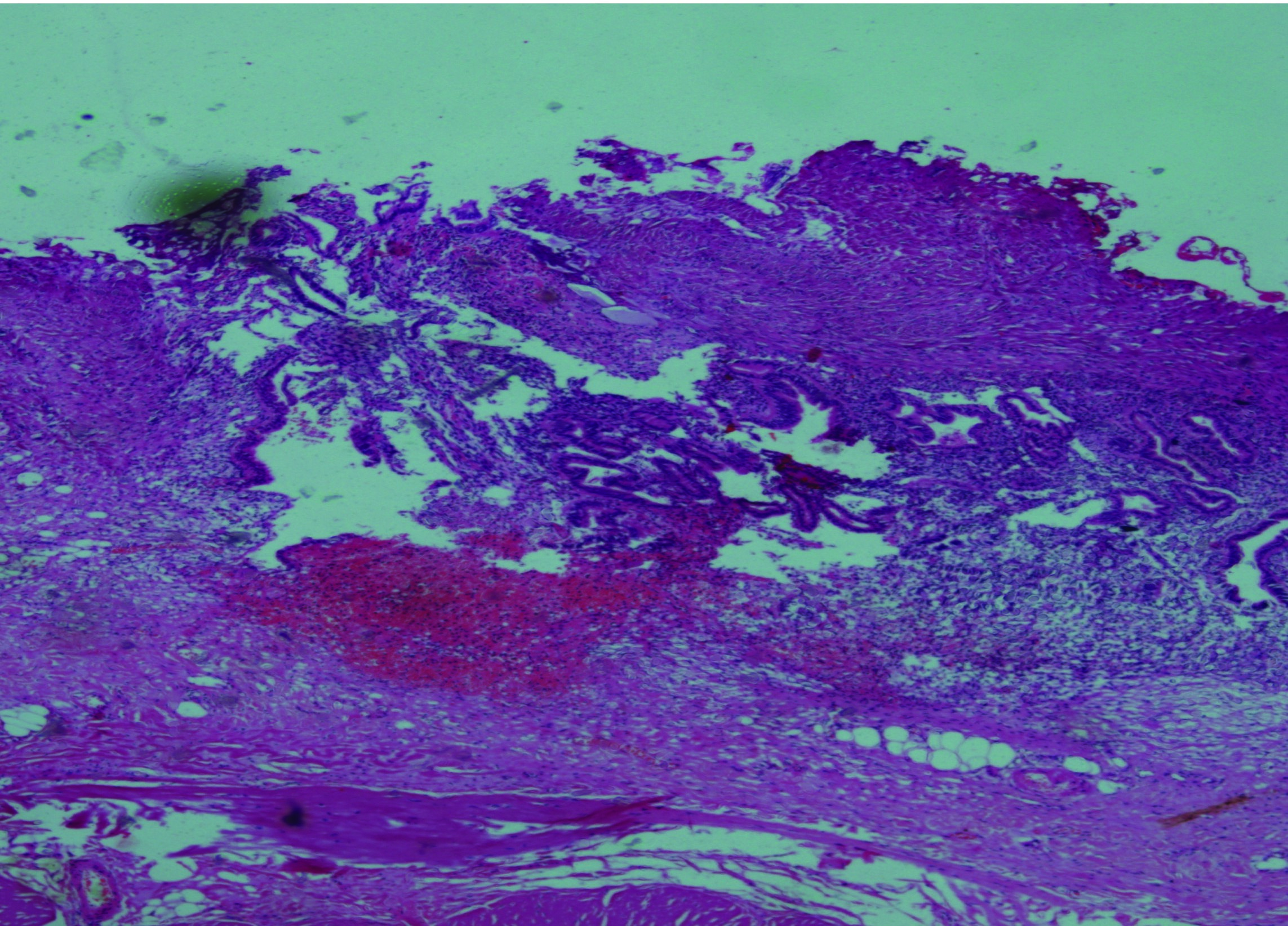
A 45-year-old female was admitted in July 2010 for dysmenorrhea of long 5years duration, P2L2. On evaluation her CA-125 was 183. Her CXR [Table/Fig-7] showed pleural effusion on right side. Ultrasound reported fibroid uterus, adenomyosis, ovarian cyst and ascites with pleural effusion on right side. CT scan reported a possibility of malignancy (apple core leison) in the distal segment of colon [Table/Fig-8] with lung metastasis. Hence colonoscopy was performed and was found to be normal. About 1.5 L of haemorragic fluid was aspirated and cytology showed haemosiderophages. Thoracoscopy findings were adhesions on pleural surface with haemorrhagic spots. Pleural biopsy reported it as endometriosis [Table/Fig-9]. She had recurrence of the same in November 2010, following which Inj DMPA was given for 8 months after which she remained asymptomatic (4 years).
Right Side Pleural Effusion.

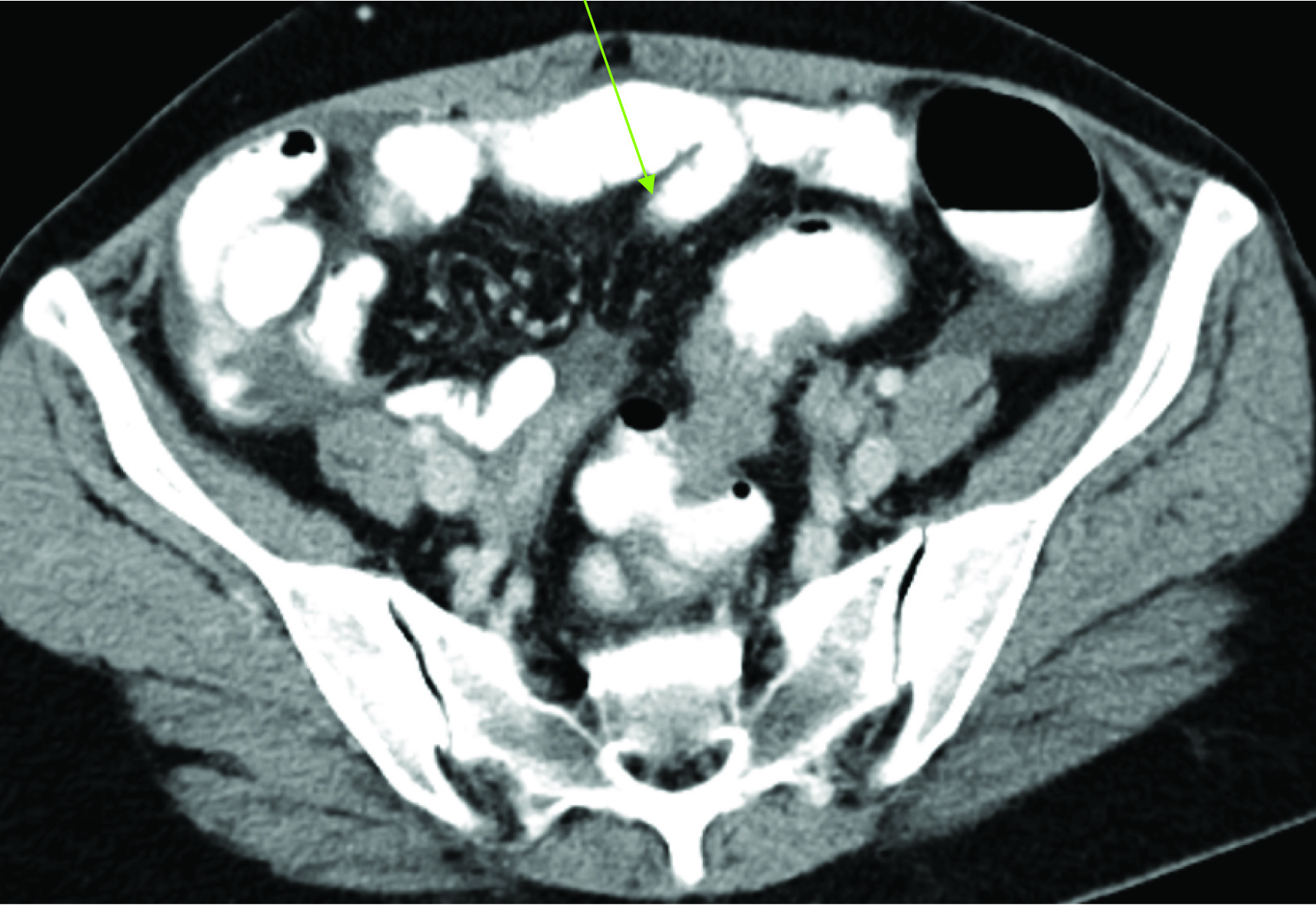
CT Abdomen-Apple Core Lesion in Sigmoid Colon.
Pleural Biopsy Suggestive of Endometriosis.
Case 6
A 23-year-old nulligravida had breathlessness; productive cough of 6months duration was referred to us in January 2014 with catamenial aggravation of the symptoms. She was treated in November 2013 for haemorrhagic pleural effusion from outside hospital. Her CT scan showed right sided massive pleural effusion. Thoracocentesis was done and drained 1.7L of chocolate coloured fluid.
Pleural fluid was showing haemorrhage, foam cells and siderophages. Ultrasound showed, endometriotic cyst (3cm), polycystic ovaries, fibroid and mild ascites. She was discharged after a week. No hormones were given.
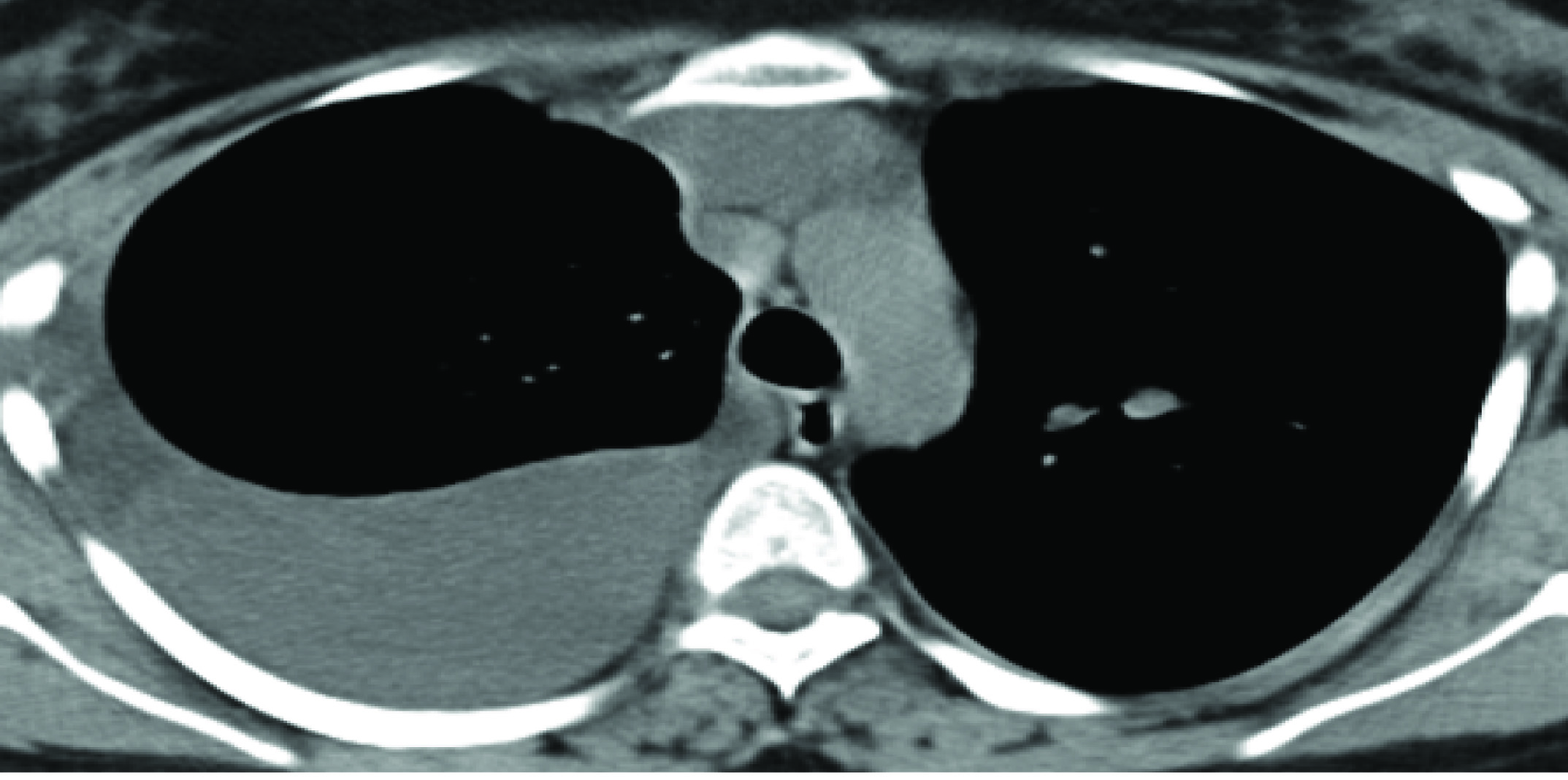
She had catamenial breathlessness and got admitted in March 2014, with right sided pleural effusion [Table/Fig-10]. Thoracoscopy was done and the findings were multiple soft cystic nodules on the diaphragm, visceral pleura and haemorrhagic spots on parietal pleura. Right lateral thoracotomy, removal of endometrial tissue and surgical pleurodesis was done. There was chocolate coloured fluid in pleural cavity, multiple fenestrations on diaphragm and brown coloured fluid on the liver surface. The diaphragm was sutured. Pleural biopsy showed stromal endometriosis. Inj GNRH was given for 3 months.
CT SCan–Right Pleural Effusion with Collapse of the Underlying Lung.
She had recurrence of symptoms in November 2014 with left sided pleural effusion [Table/Fig-11]. Decortication advised but she deferred.
CT Scan Left Pleural Effusion.
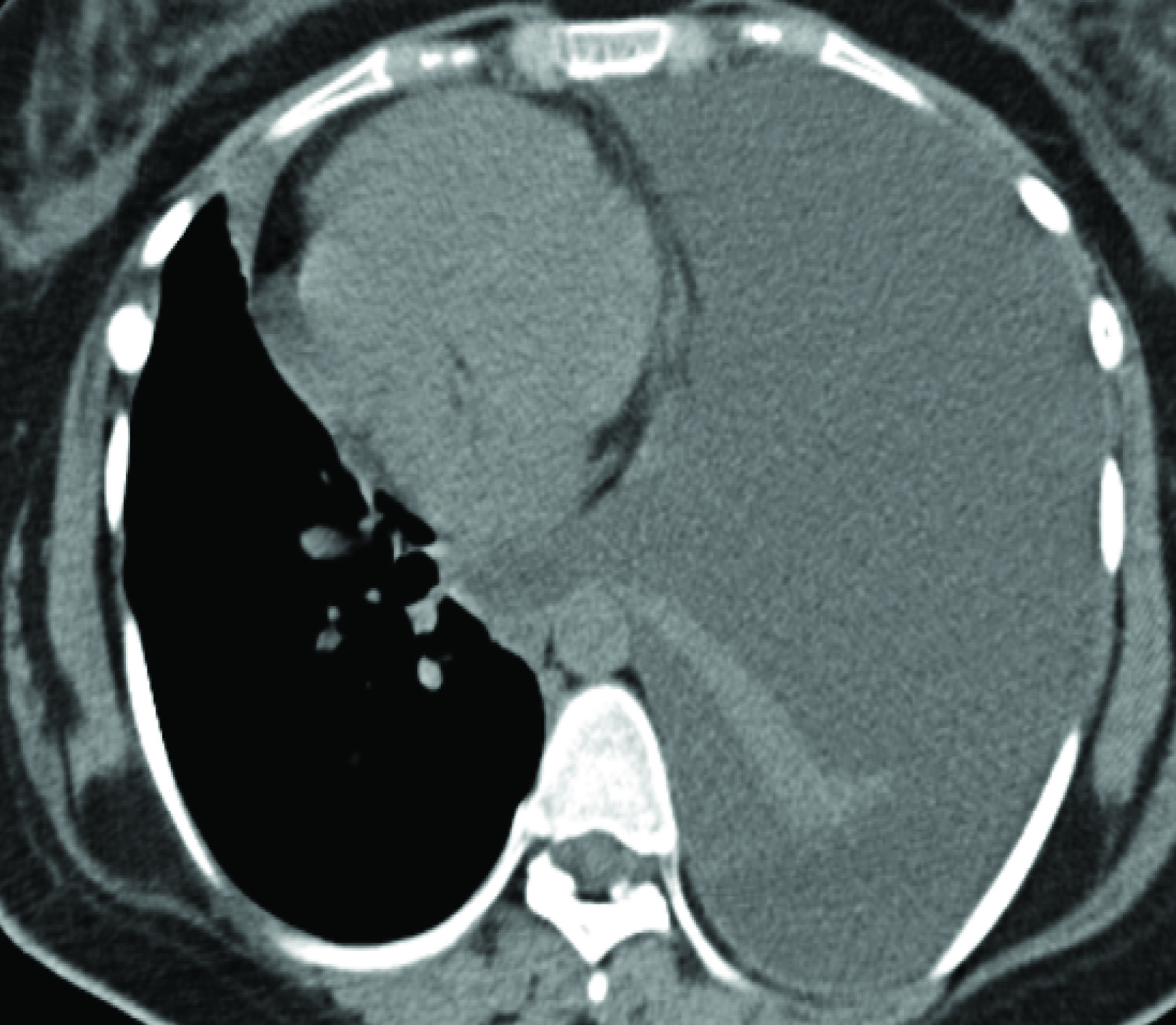
In February 2015, she developed left haemothorax. VATS was performed and the findings were adhesion of left upper lobe to chest wall, diaphragmatic sieve like defect with leak of peritoneal fluid was seen. Lung decortication with repair of the defect and TALC pleurodesis were done. She was started on daily progesterone (tab. meprate 10mg 1-0-1) and asymptomatic last 6months.
Discussion
Pelvic endometriosis affects 2-30% of women. TES is an uncommon condition [4]. It is the most frequent extra pelvic site of endometriosis [1].
Of the total women diagnosed with TES 50-85% has concomitant pelvic endometriosis. However, the percentage of women with pelvic disease who develop TES is largely unknown [2]. We had three (50%) cases of pelvic endometriosis in our study.
We had two cases (33.33%) of catamenial pneumothorax that had Marfanoid habitus. Marfanoid habitus is a risk factor for spontaneous pneumothorax [5].
In the older literature, the average duration of symptoms prior to diagnosis was 8 months, however, in subsequent reports the diagnosis has been established earlier due to increased awareness of the syndrome [2]. Our institutional case reports showed the mean duration for diagnosing TES as 15.9 months. In Inmaculade Duyos et al., study the duration of diagnosis was 15.2 months (range 3-36 months) [6].
The mean age of onset of symptoms was 36.83 years (range 23-45) in our study. The mean age was 34.5 years in Inmaculade Duyos et al., and 34 years in Aikaterini et al., studies [6,7]. Combining the results from two large reviews from 1996 and 2010, the mean age at presentation is 35 years, with a range from 19-54 years [2,8].
The symptoms of TES are typically catamenial and occur within 24-48 hours of the onset of menstruation. In a series reported by Channabasavaiah et al., chest pain is the most common symptom (90%), dyspnea (31%), haemoptysis (7%), cough (rare) and pathological entities noted in the same series were catamenial pneumothorax (73%), catamenial haemothorax (14%), catamenial haemoptysis (7%), pulmonary nodules (6%) [8]. In a small series TES, not so rare; report of three cases EKPEE et al., dyspnea was present in 100%, chest pain in about 33%, pnuemothorax and haemothorax in equal ratio, pneumothorax in 16% [9]. We had catamenial dysnea in three cases (50%), dysnea with chest pain in 1 (16%), dysmenorrhea 1 (16.7%) and chest discomfort with upper backache in the other case (16.7%). We had three cases of pneumothorax (50%) and three cases of pleural effusion (50%). Catamenial aggravation of symptoms were found in (83.3%) in our study. Aikaterini et al., had catamenial pneumothorax in all the five cases (100%) and catamenial aggravation of symptoms in two cases (40%) [7]. Inmaculade Duyos et al., had catamenial aggravation in all the five cases (100%).
Pneumothoraces are typically right sided and small to moderate in size. Haemothoraces are also usually right sided, and vary in size [10,11]. We had three cases (50%) of pneumothorax and three cases (50%) of pleural effusion. In our study the right sided presentation was about four (66.6%) and two (33.3%) were bilateral (33.3%). Aikaterini et al., and Inmaculade Duyos et al., had all the five cases (100%) on the right side. EkPEE et al., had one (33.33%) bilateral case.
About 30-50% of women with endometriosis are infertile [12]. There were two (33.3%) cases of infertility in our study. There were three cases (60%) of infertility treated patients in Inmaculade Duyo et al., study.
The CA-125 concentration was significantly higher in females with TES compared to disease free females (76.1± 49.2 vs. 16.0 ± 8.1 u/ml p <0.0001). The optimum cut off point for diagnosis of TES was 39u/ml with no false positive cases and two false negative cases. The sensitivity of CA -125 measurements in the diagnosis of endometriosis recurrence was 14.8% and the specificity was 100%. Otherwise, CA-125 is non-specifically increased in any process that irritates the mesothelial cells [13]. Only two cases CA 125 was done and was elevated in our study.
Ultrasound pelvis showed endometriosis in three (50%), adenomyosis in two (33.3%) and fibroid in one (16.7%) in our study. In a study done in 2010, 86% of 131 women who presented for treatment for symptomatic fibroids were diagnosed with concomitant endometriosis [14]. Inmaculade Duyo et al., had pelvic endometriosis four cases (80%).
The diagnosis of TES is challenging. More than 60% of affected patients may require thoracotomy or thoracoscopy as part of the diagnostic approach [8,15]. Pleural fluid cytologic examination is rarely helpful and the usual report reads presence of atypical cells [8,15]. Histology of resected lesion showed haemosiderin laden macrophages which are considered compatible or even suggestive of endometriosis [16,17]. Histopathological confirmation of pulmonary endometriosis is also difficult since both biopsy and resected specimens should be obtained just before the onset of menses. Histopathological diagnosis has been obtained in only 1/3 of the cases reported in the literature [15]. One study of 229 women (mean age 33 years) with pneumothorax, 35% of which were catamenial in aetiology, suggested that right sided pneumothorax, the presence of diaphragmatic abnormalities, relapse after surgery, and haemosderin laden macrophages on pathology were independent predictors associated with a diagnosis of thoracic endometriosis [11]. In our study three (50%) cases were proved to be endometriosis. Inmaculade Ducyo et al., had 3(60%) cases of histologically proved endometriosis.
Diagnostic procedures that have found use in TES include chest X ray (pleural effusion, opacities, nodular infiltrates), CT scan or MRI (opacities, nodules, ground glass infiltrates, thin wall cavities, bullous formation), may be non–diagnostic unless performed during menstruation), Video assisted thoracoscopy (diaphragm perforations, nodular or plaque like brown or violet endometrial deposits <1cm in size) and bronchoscopy (may reveal multiple pin like red sub mucosal lesions), and bronchial biopsy and brush cytology (may reveal endometrial cell) [15]. Bronchial artery angiography has also been used to establish the diagnosis of parenchymal endometriosis in a patient with catamenial haemoptysis [18].
The beneficial role of laparoscopy in the investigations of patients with isolated recurrent right sided chest and upper abdominal pain has been reported [19].
Performance of combined VATS and laparoscopy procedures in single session helps in assessing thoracic cavity, pelvic and sub diaphragmatic region [20]. It may be particularly helpful in cases of inconclusive VATS, which may be the result of the presence of endometriosis only in the abdominal part of the diaphragm causing catamenial phrenic nerve irritation and pain [21]. Thoracoscopy with pleural biopsy was done in 4 cases in our study. Thoracotomy was done in 1 case and VATS was done in the same patient who had left sided TES a year later.
Medical treatment often serves merely as a diagnostic tool. A positive response to medical treatment in women suspecting TES may be considered diagnostic and surgical treatment may be sought [17].
Treatment of TES is as challenging as diagnosis, and is divided into medical and surgical [15]. Suppression of the ectopic endometrium by interfering with ovarian oestrogen secretion by OCP, PROGESTINS, DANAZOL or GnRH analogues has been widely used [2,22]. Hormonal treatment appears to be more effective for catamenial haemoptysis than catamenial pneumothorax.
GnRH agonist have been found to be more effective in controlling recurrences of catamenial pneumothorax, particularly when used for prolonged period’s upto 1year [23,24]. However, pneumothorax and haemothorax recur in greater than 50% despite hormonal therapy, suggesting that endometrial implant regression is not complete and or that recurrent embolization from pelvic foci continues [25]. Occasional patients have symptoms refractory to all of the above modalities. These patients respond to hysterectomy with bilateral salpingoophorectomy [26].
One of our patients diagnosed as TES, was given INJ DMPA but she had recurrence within 6months of treatment and underwent hysterectomy with bilateral salpingoophorectomy. Again she had recurrence 6 months after hysterectomy with bilateral salpingoophorectomy and was given inj GnRH for 1 year. Now she is asymptomatic. Endometriosis related Pneumothorax has been revealed even (6 years) after hysterectomy performed for pelvic endometriosis [16].
Initial surgical procedures include supportive oxygen, observation and rest if lung collapse is small. Medium to large collapse may require manual aspiration or chest tube. A second occurrence may be treated more aggressively with a sclerosing agent. Literature shows that pleurodesis used as a singular treatment without other surgical repair or hormonal therapy has a higher failure rate [27,28]. We did pleurodesis in 3 cases (2 with betadine and 1 with TALC). One patient had recurrence 6months after pleurodesis in 2012.
A 23-year-old with 3 recurrences was treated by TALC pleurodesis along with lung decortication. She was started on progesterone and is on follow up since 6 months.
Recurrences at 6 months and 12 months were 50% to 60% after medical treatment and 5% to 25% after surgical treatment [2].
Pleurodesis, especially in younger patient, should always be carried out concomitantly with VATS exploration. Hormonal treatment with GnRH agonist seems to improve the outcome [1]. In our study two cases which had multiple recurrences were disseminated endometriosis affecting both lungs. Multiple mode of therapy was done in both cases.
Major surgical procedures done are removal of endometrial implants, repair of diaphragmatic fenestrations, and abrade pleural surfaces, remove the pleura or remove the ovaries. Add back oestrogen therapy is withheld for several months, as endometriosis can recur with oestrogen therapy, even if oophorectomy done [29].
TES was strongly associated with severe pelvic endometriosis and high rate of infertility. Majority (6/7) of the patients had symptoms of pelvic endometriosis (dysmenorrhoea, dyspareunia and pelvic pain) beginning at a mean median age of 16years. Of these 4 received treatment with OCP from 18.5 years before establishment of the diagnosis. Mean age of surgery was 31years. There is a considerable delay between onset of symptoms and definitive diagnosis. Even if TES is diagnosed, it need not be necessary that pelvic endometriosis is present at the same time; it may appear later [30]. Hence prompt diagnosis and treatment with hormones for suppression of ectopic endometrial glands and long term follow up is needed.
Limitations
Although our study has Limitations, study population was small (6 in number), due to rarity of the case. Management strategies were different in each case. This may be due to rarity of the disease, varying parity, lack of awareness and due to recurrence of the disease.
Conclusion
TES is virtually a diagnosis of exclusion established on clinical grounds. Early diagnosis should lead to a time specific therapy and decreased morbidity. Combined effort of thoracic surgeon, gynaecologist and pulmonologist helps in diagnosing and treating TES at the earliest. Apart from medical management, VATS and laparoscopy are useful surgical procedures in diagnosing as well as treating TES along with the hormones.
[1]. Nezhat C, Buescher E, Paka C, Hajhosseini B, Hilaris GE, Thoracic Endometriosis SyndromeWorld Clin Obstet Gynecol 2011 1(2):228-38. [Google Scholar]
[2]. Joseph J, Sahn SA, Thoracic endometriosis syndrome: new observations from an analysis of 110 casesAM J Med 1996 100:164-70. [Google Scholar]
[3]. Definition from merck source.com (Dorlands Medical Dictionary) Wikipedia [Google Scholar]
[4]. GUO SW, Wang Y, The prevalence of endometriosis in women with chronic pelvic painGynecol Obstet Invest 2006 62:183 [Google Scholar]
[5]. Cone D, Brice JH, Delbridge TR, Brent J, Myers Emergency Medical Services: Clinical Practice and systems oversight2 volumes et 2015 [Google Scholar]
[6]. Duyos I, López-Carrasco A, Hernández A, Zapardiel I, Management of thoracic endometriosis:single institution experienceEuropean Journal of Obstetrics & Gynaecology and Reproductive Biology 2014 178:56-59. [Google Scholar]
[7]. Visouli AN, Catamenial Pneumothorax: a rare entity? Report of 5cases and review of literatureJ Thoracic Dis 2012 4(suppl 1):17-31. [Google Scholar]
[8]. Channabasavaiah AD, Joseph JV, Thoracic endometriosis: revisiting the association between clinical presentation and thoracic pathology based on the thoracoscopic findings in 110 patientsMedicine (Balitimore) 2010 89:183 [Google Scholar]
[9]. Ekpee EE, Bassey EA, Umanah IN, Thoracic Endometriosis Syndrome, Not so rare; report of 3casesCase study and case report 2013 3(2):95-102. [Google Scholar]
[10]. Alifano M, Jablonski C, Kadiri H, Catamenial and non catamenial, endometriosis-related or non endometriosis-related pneumothorax referred for surgeryAm J Respir Crit Carev Med 2007 176:1048-53. [Google Scholar]
[11]. Legras A, Mansuet-Lupo A, Rousset–Jablonski C, Pneumothorax in women of child-bearing age: an update classification based on clinical and pathological findingsChest 2014 145:354 [Google Scholar]
[12]. Holoch KJ, Lessey BA, Endometriosis and infertilityClin Obstet Gynecol 2010 53:429-38. [Google Scholar]
[13]. Le Pimpec Barthes F, Riquet M, Value of cancer antigen 125 for diagnosis of pleural endometriosis in females with recurrent pneumothoraxEuropean Respiratory Journal ERJ 2008 31(1):140-42. [Google Scholar]
[14]. Hughes EG, Fedorkow DM, Collins JA, A qualitative overview of controlled trials in endometriosis associated infertilityFertil Steril 1993 959:963-70. [Google Scholar]
[15]. Augoulea A, Lambrinoudaki I, Chirstodoulakos G, Thoracic Endometriosis SyndromeRespiration 2008 75:113-19. [Google Scholar]
[16]. Nezhat C, King LP, Paka C, Bilateral thoracic endometriosis affecting the lung and diaphragmJSLS 2012 16:140-42. [Google Scholar]
[17]. Triponez F, Alifano M, Bobbio A, Endometriosis-related spontaneous diaphragmatic ruptureInteract Cardio Thorac Surg 2010 11:485-87. [Google Scholar]
[18]. Hope-Gill B, Prathibha BV, Catamenial haemoptysis and clomiphene citrate therapyThorax 2003 58:89 [Google Scholar]
[19]. Redwine DB, Diaphragmatic endometriosis diagnosis, surgical management, and long-term results of treatmentFertil Steril 2002 77:288 [Google Scholar]
[20]. Nezhat C, Crowgey SR, Garrison CP, Surgical treatment of endometriosis via laser laparoscopyFertil Steril 1986 45:778-83. [Google Scholar]
[21]. Nezhat C, Seidman DS, Nezhat F, Laparoscopic surgical management of diaphragmatic endometriosisFertil Steril 1998 69:1048-55. [Google Scholar]
[22]. Marshall MB, Ahmed Z, Kucharczuk JC, Catamenial puemothorax; optional hormonal and surgical managementEur J Cardiothoracic Surg 2005 27:662 [Google Scholar]
[23]. Tripp HF, Thomas LP, Obney JA, Current therapy of catamenial pneumothoraxHeart Surg Forum 1998 1:146-49. [Google Scholar]
[24]. Slabbynck H, Laureys M, Impens N, De Vroey P, Schandevyl W, Recurring catamenial pneumothorax treated with a GnRH analogueChest 1991 100:851 [Google Scholar]
[25]. Alifano M, Roth T, Broet SC, Catamenial pneumothorax: a prospective studyChest 2003 124:1004-08. [Google Scholar]
[26]. Rivas de Andres JJ, Jimenez Lopez MF, Lopez Rodo LM, Perez Trullen A, Torres Lanzas J, Guidelines for the diagnosis and treatment of spontaneous pneumothoraxJ Arch Broncneumol 2008 44(8):437-48. [Google Scholar]
[27]. Ciriaco P, Negri G, Libretti L, Carretta A, Melloni G, Casiraghi M, Surgical treatment of catamenial pneumothorax: A Single Centre ExperienceInteract Cardiovasc Thorac Surg 2009 8(3):349-52.Epub 2008 Dec 16 [Google Scholar]
[28]. Leong AC, Coonar AS, Lang-Lazdunski L, Catamenial pneumothorax: surgical repair of the diaphragm and hormone treatmentAnn R Coll Surg Engl 2006 88:547 [Google Scholar]
[29]. Gamaleidein H, Tetzlaff JE, Whalley D, Anesthetic implications of thoracic endometriosisJ Clin Anesth 2002 14(1):36-8. [Google Scholar]
[30]. Soriano D, Thoracic endometriosis syndrome is strongly associated with severe pelvic endometriosis and infertilityThe Journal of Minimally Invasive Gynecology 2012 19(6):772-74. [Google Scholar]