Leukaemic Transformation of Multiple Myeloma in Post Chemotherapy Remission Phase
Palak Agarwal1, Prachi Nayak2, Premala Anthony Singh3, Bal Krishna Mishra4
1 Department of Pathology, Kriti Scanning Centre Pvt Ltd, Allahabad, India.
2 Department of Pathology, Anoop Labs Pvt Ltd, Allahabad, India.
3 Department of Pathology, Anoop Labs Pvt Ltd, Allahabad, India.
4 Department of Medicine, Kriti Scanning Centre Pvt Ltd, Allahabad, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Palak Agarwal, 55-B, Lowther Road, Near Medical College Allahabad-211001, India.
E-mail: palak_aga3@yahoo.com
Plasma cell leukaemia is diagnosed when plasma cells are >20% in the peripheral blood. Plasma cell leukaemia may be present at the time of diagnosis (primary plasma cell leukaemia) or may evolve from multiple myeloma (secondary plasma cell leukaemia). We report case of a 62-year-old male who was diagnosed with multiple myeloma. He was treated with combination of prednisolone, melphalan and thalidomide. After 6 years he had Worsening of symptoms and also developed a scalp swelling. The swelling was diagnosed as plasmacytoma on fine needle aspiration cytology and confirmed on histopathology. Complete haemogram showed-Haemoglobin - 8g/dl, Total Leucocyte Count – 4300/μl, Differential leucocyte count - Neutrophil-40%, Lymphocyte-28%, Eosinophil-01%, Monocyte-10%, Atypical cells-21%, Platelet count- 1.5 lacs/μl. Peripheral blood showed rouleaux formation and plasma cells. Serum protein electrophoresis revealed an M spike (3.26 g/dl). So, patient was diagnosed as secondary plasma cell leukaemia. Weekly bortezomib and dexamethasone combination chemotherapy was given to the patient. Patient is on monthly follow up. Here we present a detailed case history of this patient.
Bortezomib, Bone Marrow, Hemogram, SPEP
Case Report
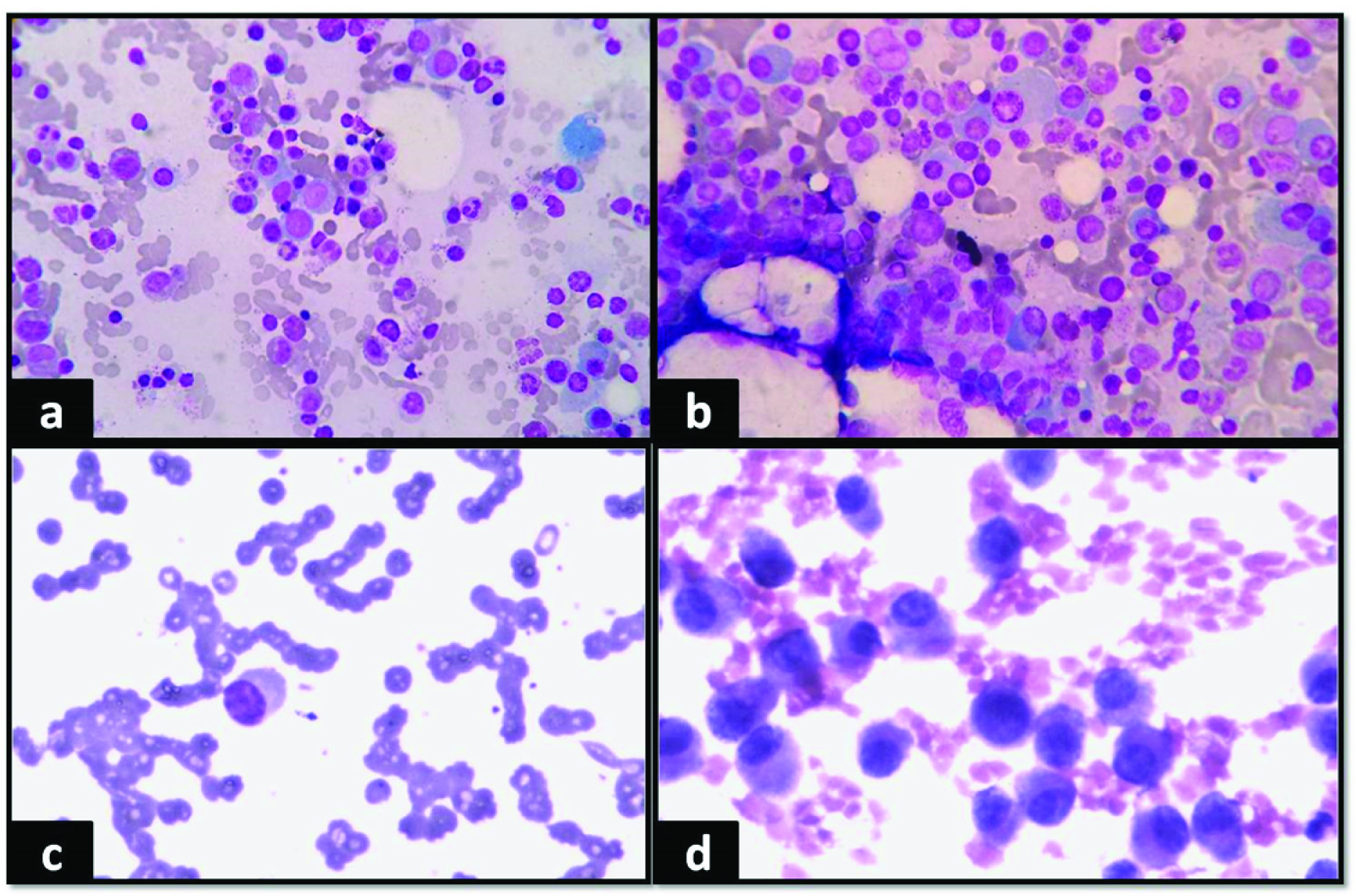
A 62-year-old male presented in the oncology outpatient department with complaints of headache, bodyache, weakness and anorexia for 2 months. On routine haemogram there was persistent bicytopenia with normocytic normochromic anaemia (Haemoglobin (Hb)- 9 g/dl, Total Leucocyte Count(TLC)- 2180/μl, Differential Leucocyte Count (DLC)- Neutrophil-64%, Lymphocyte-32%, Eosinophil-2%, Monocyte-2%, Platelet count- 1.5 lacs/μl). Digital X–ray spine (lateral view) showed osteopenia. Bone marrow examination was done and smears were particulate. There was increase in number of plasma cells constituting about 48% of all nucleated cells. Few binucleate and immature forms were also seen [Table/Fig-1a&b]. There was mild suppression of haemopoiesis. An impression of Multiple myeloma (MM) was given. Serum protein electrophoresis(SPEP) and immunofixation were advised, which showed- total protein-7.2g/dl, albumin-3.23g/dl, α1-0.38g/dl, α2-1.36g/dl, β-0.96g/dl, γ-1.27g/dl, A/G ratio-2.54 and M spike-0.92g/dl. Immunofixation- IgG Kappa band positivity in neoplastic plasma cells [Table/Fig 2a]. β2 microglobulin- 2.35mg/l, urea- 60mg/dl and fasting blood sugar- 151mg/dl. [International Staging System Score- II].
(a) Giemsa, 40x Plasmablast. (b) Plasma cells in bone marrow aspirate. (c) Giemsa, 40x, Peripheral blood smear showing plasma cells and rouleaux formation. (d) H&E, 40x, Plasmacytoma in scalp swelling.
(a) SPEP 2009 (M spike- 0.92g/dl). (b) SPEP 2015 showing increase in M spike (3.26g/dl).
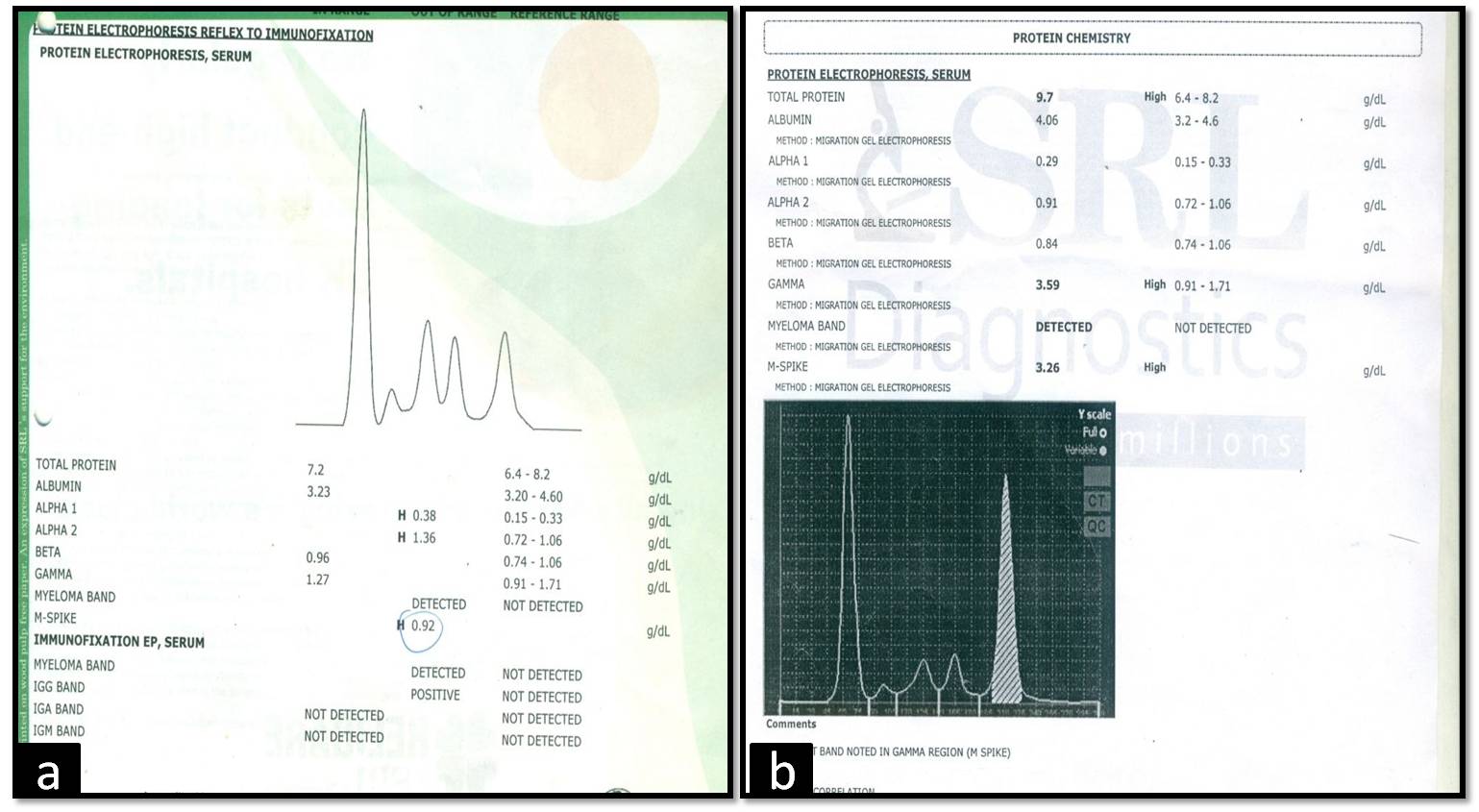
The patient was given prednisolone, melphalan, and thalidomide as chemotherapy because he refused autologous bone marrow transplantation. He was on regular follow up and his general condition improved. There was decrease in M spike in subsequent SPEP. After 6 years, he had Worsening of symptoms and also developed a scalp swelling. Complete haemogram, SPEP and Fine Needle Aspiration (FNA) of the swelling were advised. SPEP showed- total protein-9.7g/dl, albumin-4.06 g/dl, α1-0.29 g/dl, α2-0.91 g/dl, β-0.84 g/dl, γ-3.59 g/dl, A/G ratio-1.13 and M spike-3.26 g/dl [Table/Fig-2b]. Haemogram- Hb-8g/dl, TLC- 4300/μl, DLC- Neutrophil-40%, Lymphocyte-28%, Monocyte-10%, Eosinophil-01%, Atypical cells- 21%, Platelet count- 1.5 lacs/μl. Peripheral blood smear showed rouleaux formation with atypical cells of varying size with pale blue cytoplasm, large eccentric nucleus and perinuclear hof [Table/Fig-1c]. Patient was diagnosed as secondary Plasma Cell Leukaemia (sPCL).
FNA smears from scalp swelling showed dispersed population of plasma cells with eccentric nucleus, perinuclear hof and basophilic cytoplasm [Table/Fig-1d]. Background showed lymphocytes and blood. A FNA diagnosis of plasmacytoma was given. It was confirmed on histopathology.
The patient was treated with weekly 2mg bortezomib intravenous injections and 40mg oral dexamethasone chemotherapy leading to decrease in number of plasma cells in the peripheral blood. A total of 11 cycles were completed. Patient had symptomatic relief and he is now on regular monthly follow up.
Discussion
PCL is a rare disease with a prevalence of 1-2% of all hematologic malignancies [1] and 2-3% of all plasma cell dyscrasias [2]. Plasma cell neoplasms result from expansion of a clone of immunoglobulin secreting terminally differentiated B cells that secrete monoclonal immunoglobulin. The diagnostic Criteria for PCL is: 1) presence of a circulating clonal plasma cell count of > 2000/μL (if TLC is >10,000/μl); or 2) presence of >20% circulating plasma cells (if TLC is <10,000/μL) as in our case [3]. PCL can arise either de novo {primary PCL(pPCL)} or in patients with pre-existing MM (sPCL), as seen in 12% cases [4]. Patients with sPCL have a worse prognosis than those with pPCL. sPCL appears to have a male: female ratio of 3:2 and a median age of 50 years at diagnosis [1].
In addition to peripheral blood and bone marrow, the neoplastic plasma cells may be found in extramedullary tissue such as liver and spleen and in body cavity fluids. Most clinical signs of MM are observed in PCL, although osteolytic lesions and bone pain are less frequent (more common in sPCL) and lymphadenopathy, organomegaly and renal failure are often present (more common in pPCL) [3,5,6].
Plasma cells are morphologically easy to identify in bone marrow, however, they are often missed on peripheral blood smear examination. The cytologic characteristics of neoplastic plasma cells is variable, but often, they are small with little cytoplasm and resemble plasmacytoid lymphocytes [3].
The reason for leukaemic transformation in MM is unknown. In MM, malignant plasma cells are tightly dependent on the bone marrow microenvironment for their growth and survival; however, in PCL they disseminate into peripheral blood. This is caused by different expression of adhesion molecules and chemokine receptors sustained by several molecular aberrations [7]. Literature says that CD56 antigen has an important role in anchoring plasma cells to bone marrow stroma. However, most of the plasma cells in MM aberrantly express CD56. Pellat DC et al., have reported that the neoplastic cells of PCL (primary or secondary) do not express or weakly express CD56 in contrast to patients with MM [8]. Lack of CD56 may be associated with More extramedullary disease [8].
Presence of plasma cells can be confirmed by flowcytometric evaluation demonstrating strong positivity for CD38 and CD138. Plasma cells in MM aberrantly express: HLADR, CD117 and CD20 [3]. Soluble CD95 levels have been observed to be elevated in PCL. The lack of (or weak) expression of CD56 is a characteristic feature of PCL [9].
Katodritou et al., demonstrated that patients treated with bortezomib versus conventional therapies had a higher survival of 13 months versus 2 months and also high response rates [10]. The proteasome inhibitor bortezomib has shown multifactorial biological effects on bone marrow microenvironment and myeloma cells. It suppresses the expression of adhesion molecule and is anti-angiogenic [7]. Magalhaes et al., reported a case of sPCL. The patient achieved complete remission on treatment with conventional chemotherapy and bortezomib but later on she died due to sepsis [11].
Conclusion
In conclusion, as diagnosis of PCL has considerable implications on the treatment and prognosis, more awareness and earlier recognition of the characteristic smear findings is of significance to improve the therapeutic outcome, increase survival of patients and attempt to understand the behaviour of this rare and aggressive neoplasm.
[1]. Caballero MA, Flores DL, Gutierrez RR, Secondary plasma cell leukaemia in a patient with light chains multiple myeloma in post chemotherapy remission phaseJournal of Applied Hematology 2014 5(3):111-14. [Google Scholar]
[2]. Rotaru I, Gaman G, Dumitrescu D, Foarfa C, Secondary plasma cell leukaemiaRom J Morphol Embryol 2012 53(4):1073-76. [Google Scholar]
[3]. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, WHO classification of tumors of haematopoietic and lymphoid tissues 2008 LyonIARC [Google Scholar]
[4]. Moghaddam N, Taheri D, Naimi A, Taghizadeh F, Taheri S, An unusual presentation of plasma cell leukaemia with undiagnosed multiple myelomaIran J Pathol 2007 2:77-79. [Google Scholar]
[5]. Buskard NA, Boyes DA, Grossman L, Plasma cell leukaemia following treatment with radiotherapy and melphalanCan Med Assoc J 1977 117:788-89. [Google Scholar]
[6]. Reimer RR, Hoover R, Fraumeni JF, Young RC, Acute leukaemia after alkylating agent therapy of ovarian cancerN Engl J Med 1977 297:177-81. [Google Scholar]
[7]. Simeon V, Todoerti K, Rocca F, Caivano A, Trino S, Lionetti M, Molecular Classification and Pharmacogenetics of Primary Plasma Cell Leukaemia: An Initial Approach toward Precision MedicineInt J Mol Sci 2015 16:17514-34. [Google Scholar]
[8]. Pellat DC, Barille S, Jego G, Puthier D, Robillard N, Pineau D, The absence of CD56 (NCAM) on malignant plasma cells is a hallmark of plasma cell leukaemia and of a special subset of multiple myelomaLeukaemia 1998 12:1977-82. [Google Scholar]
[9]. Gertz MA, Buadi FK, Plasma cell leukaemiaHaematologica 2010 95(5):705-07. [Google Scholar]
[10]. Katodritou E, Terpos E, Kelaidi C, Kotsopoulou M, Delimpasi S, Treatment with bortezomib-based regimens improves overall response and predicts for survival in patients with primary or secondary plasma cell leukaemia: Analysis of the Greek myeloma study groupAm J Hematol 2014 89:145-50. [Google Scholar]
[11]. Magalhaes LG, Almeida JC, Araujo RC, Silva JF, Brandao CAF, Medeiros PL, Plasma Cell Leukaemia Remains Like an Incurable Disease? A Rare Presentation of an Aggressive Case of Secondary Plasma Cell LeukaemiaJ Leuk 2015 2(4):1-3. [Google Scholar]