Diffuse Large B Cell Lymphomas (DLBCL) encompasses a heterogeneous group of tumors that together constitute the commonest of all Non Hodgkin Lymphoma (NHL) and the proclivity of DLBCL to oral cavity is unknown. They mostly arise from soft tissues as asymptomatic lesions, mostly without ‘B’ symptoms and involvement of jaw bones is uncommon. Most studies and case reports of oral DLBCL’s are based on, manifestation of primary extra-nodal disease or a component of a disseminated disease process involving regional lymph nodes. Many investigators have proposed that patients with this cell type who maintain a complete response for 24 consecutive months are cured because late relapses seldom occur. With advances in treatment modalities, many patients with NHL become long-term survivors and the risk of relapses or second tumors are of growing concern. We present a case of DLBCL which relapsed after five years of initial lesion in a 41 year old female patient and presented as a nonspecific bilateral anterior maxillary radiolucency. DLBCL usually express pan-B markers with small percentage expressing T-cell markers. Few rare cases of DLBCL have shown CD3 expression, which is a most sensitive T-cell marker which was focally expressed in the present case.
Immunohistochemistry, Intrabony, Non-Hodgkin’s lymphoma, Radiolucency, Swelling
Case Report
A female patient aged about 41 years reported to Meghana Institute of Dental Sciences with a complaint of pain and bilateral swelling which was insidious in onset and gradually increased to the present size since one month. She presented with a past history of vague pain and discomfort in right upper front teeth since four months and underwent root canal treatment for right maxillary lateral incisor (12) by a private dental surgeon with antibiotics and pain medication, who diagnosed it as periapical abscess. Her symptoms did not subside and noticed a small swelling on right anterior labial sulcus of maxilla initially and later on left. There was no relief from pain and hence she approached the institution. Extra-oral examination was noncontributory and no lymph nodes were palpable. On intra-oral examination bilateral swelling was evident in the labial sulcus adjacent to labial frenum in relation to apical region of 13, 12, 11 and 22, 23. On palpation there was a smooth surfaced non-tender swelling, firm in consistency. Periapical radiograph revealed a lytic lesion apical to 12, 13 [Table/Fig-1] and Orthopantomogram (OPG) was advised that revealed bilateral ill-defined periapical radiolucencies in relation to 13, 12 and 22, 23. [Table/Fig-2]. The teeth in the region were not mobile, vital except 12 due to incomplete root canal treatment. Thorough examination was conducted and a detailed history was taken. Family history was noncontributory and on insistence she revealed inguinal lymph node enlargement five years back, for which she was treated. Her medical records were reviewed which revealed Stage-I, Non Hodgkins Lymphoma (Large B cell) of inguinal lymph nodes. At a local cancer institute CHOP regimen (Cyclophosphamide, Doxorubicin, Vincristine, Predinosone) six cycles with three weeks interval was given. Follow up of two years was uneventful. Considering her clinical history, a provisional diagnosis of relapse of NHL was made and differential diagnoses of periapical inflammatory lesions or odontogenic lesion were considered. Serologic investigations for HIV, Hepatitis virus surface antigens (HbsAg) were non-reactive. Blood picture was within normal limits.
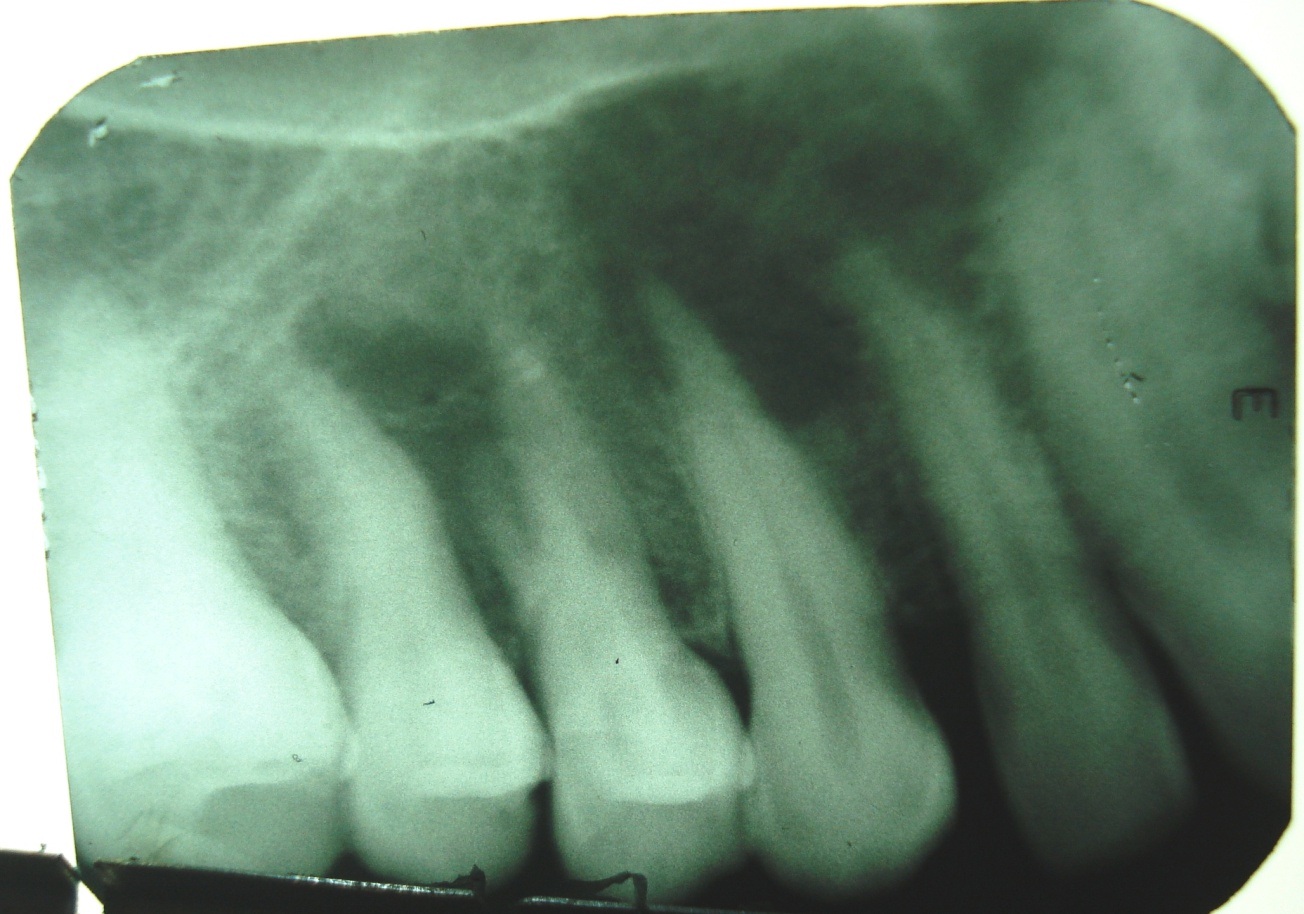
Intraoral periapical radiograph showing ill-defined periapical radiolucency involving 12, 13.
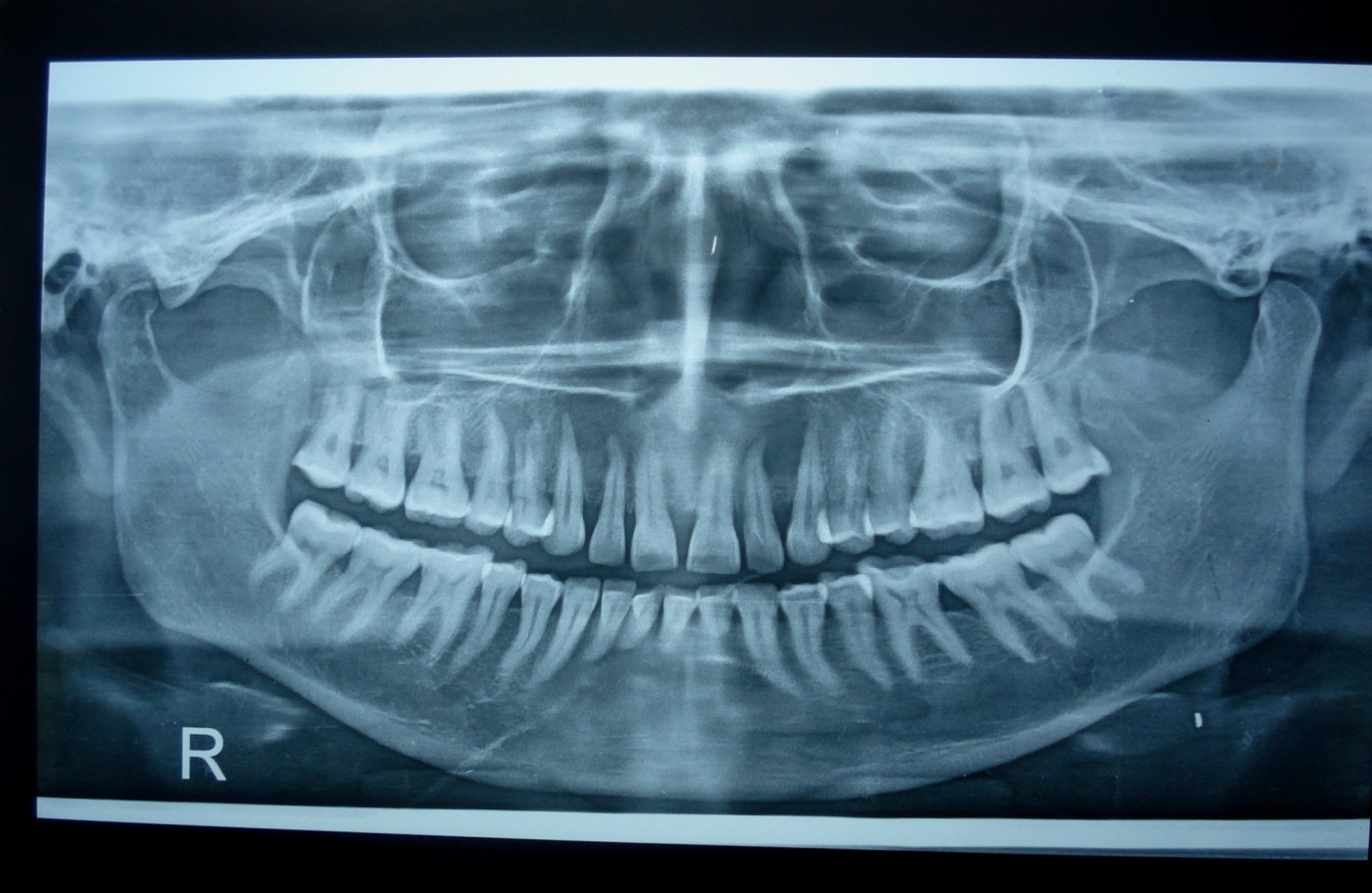
Orthopantomograph (OPG) revealing bilateral ill-defined periapical radiolucency involving 12, 13 and 22, 23.
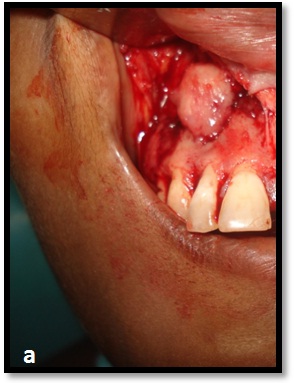
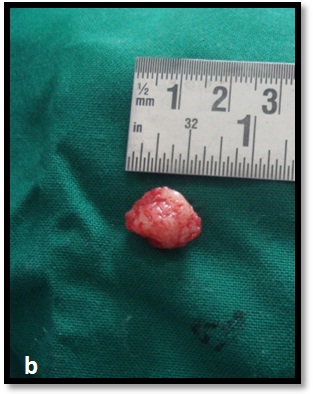
With patients consent excisional biopsy was performed. The site was exposed under local anesthesia with elevation of the mucoperiosteal flap, which exhibited perforation of buccal plate and exposure of lesional tissue. The entire lesion was excavated and was submitted for histopathological diagnosis to Panineeya Institute of Dental Sciences and Research Centre. Gross specimen showed a white fleshy mass with irregular surface of size 1cm x 2cm [Table/Fig-3a, 3b]. The biopsy specimen revealed sheets of predominantly medium to large size mononuclear cells with pleomorphic oval to round nucleus, fine chromatin, containing prominent nucleoli resembling, centroblasts and was diagnosed as NHL [Table/Fig-4a, 4b]. Immunohistochemistry (IHC) showed Leucocyte Common Antigen (LCA) positive [Table/Fig-5]; CD20 (B cell) positive [Table/Fig-6]; Bcl-2 positive [Table/Fig-7]; Pancytokeratin (CK) negative, CD5 (T cell) negative; proliferation index with Ki-67 showed<50% [Table/Fig-8]. There was heterogeneous expression of CD3 (T cell) marker at focal areas [Table/Fig-9a, 9b]. The final diagnosis was NHL-Diffuse Large B-Cell Type-High Grade. The patient was referred to cancer institute where chest X-ray, ultrasound of abdomen, bone scan and bone marrow biopsy did not reveal any abnormality and confirmed the lesion to be localized. Chemotherapy was advised by the oncologist with CHOP regimen (six cycles). However at the end of the third cycle of CHOP, a nodule in the right breast was noticed. The histopathological diagnosis revealed similar lesion of DLBCL. After three weeks she succumbed to systemic complications.
Excisional biopsy revealed bone perforation with an irregular white mass.
Gross specimen measuring 1cm x 2cm white fleshy lesional tissue.
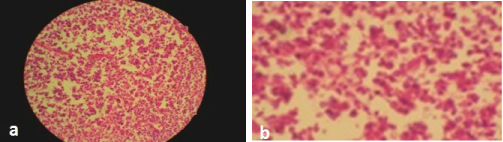
Photomicrograph H&E stain, (4x) showing medium to large mononuclear cells with pleomorphic oval to round nucleus, fine chromatin, containing prominent nucleoli resembling, centroblasts, b- High power (40x) view, centroblasts
Photomicrograph IHC (4x), neoplastic cells showing positivity to LCA (Leukocyte Common Antigen).
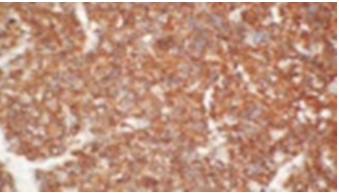
Photomicrograph IHC (4x), neoplastic cells showing strong positivity to CD20.
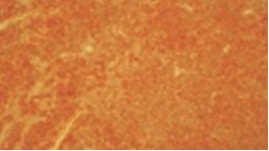
Photomicrograph IHC (4x), neoplastic cells showing strong membranous BCL2 positivity.
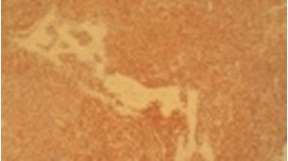
Photomicrograph IHC (40x), nuclear positivity of Ki-67 showing <50%.
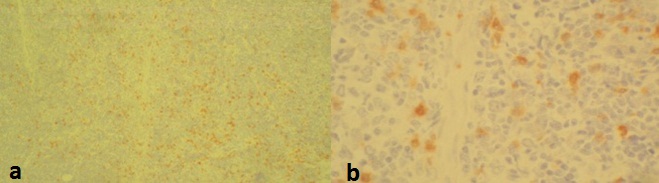
Photomicrograph IHC (4x), focal areas of neoplastic cells showing CD3 positivity, b) High power view (40x).
Discussion
Non-Hodgkin’s Lymphomas (NHL) are a heterogeneous group of lymphoproliferative malignancies that are much less predictable than Hodgkin’s and have a far greater predilection to disseminate to extranodal tissues [1]. In the head and neck region, NHL has been observed in the Waldeyer’s ring, oral mucosa, salivary glands, paranasal sinuses, laryngeal tissue, and osseous structures [2]. In the lymphomas of oral region, the prevalent site is tonsil followed by parotid, tongue, palate, gum and lip. Oral involvement frequently is part of widespread disease and may also involve the head and neck region [3]. NHL mostly arises from soft tissues as asymptomatic, soft-elastic lesions, frequently without ‘B’ symptoms. The maxilla is more frequently involved than mandible [1,4].
Oral NHLs are shown to be predominantly of B-cell lineage and are of DLBCL subtype. The greater predominance of DLBCL in the oral cavity is unknown but has been explained as a natural inclination for this site [1,3,4]. It may occur at all ages, but generally in patients more than 40 years of age and females account for 64% of the total [5]. The age of the patient in the presented case was 36 years at her initial inguinal node DLBCL with a recurrence after five years in maxilla. DLBCL has been reported in the oral cavity in the buccal mucosa, hard palate, gingiva and the maxillary vestibule was a common site for the occurrence of soft tissue tumors [1,6]. The primary intrabony location of DLBCL is uncommon and have been reported in literature in the maxilla and mandible [6–8]. The most common presentations are pain (55%), swelling of the jaw (58%), and mental numbness or dysesthesias (20%). The most less frequent complaints include poor dentition, loosening of the teeth or persistent swelling and pain after dental extraction [8]. They are often misdiagnosed which are similar to an odontogenic tumor, cyst, or infection. Radiographically it appears as a radiolucent area that may mimic endodontic lesion, periodontal pathology, or odontogenic cyst or tumor [1, 4, 6, 7]. The initial wrong diagnosis is followed by multiple extractions and/or root canal treatments as in the present case. They may also show sclerosis, resorption of the root of the teeth, destruction of the buccal cortex, or pathological fractures [8].
The case reports and studies of oral DLBCL’s are reported, as a manifestation of primary extra-nodal disease or a component of a disseminated disease process involving regional lymph nodes. There are not many reports regarding the relapse cases in jaws. This rare case of DLBCL of maxilla presented as initial manifestation of late relapse after five years, as an intra-osseous extranodal lymphoma. There was no nodal involvement or bone marrow involvement at relapse which led to late diagnosis which significantly deteriorated prognosis. DLBCL corresponds to a group of lymphoid malignancies composed of large cells with vesicular nuclei, prominent nucleoli, basophilic cytoplasm and a usually high proliferation rate [9]. DLBCL is considered an aggressive yet treatable neoplasm with a variable clinical course. Long term disease free survival is shown to be 50% and can reach 80% when lymphoma is localized at diagnosis. Some patients relapse in the first 2-3 years following treatment but relapses that occur after five years are considered rare events [9–11]. Most investigators have recognized that practically all patients with this cell type who are able to attain and maintain a complete response for 24 consecutive months are cured as late relapses are uncommon [10]. A late relapse occurred in 3.6% of patients with DLBL treated in Lyon from 1985 to 2003. In this study late relapse patients had initial early stage disease in 67% patients, 82% had low or low-intermediate International Prognostic Index (IPI) score and 65% patients had extranodal involvement with 50% primary extranodal involvement. The extranodal sites involved in relapse patients were mainly bone-marrow, bone, tonsil and tongue in oral cavity [9]. Patients with DLBL relapse usually had the same histology and few cases relapsed as indolent lymphoma following initial DLBL and no information regarding evolution is available. The patients who relapsed with DLBL had poor outcome than with indolent histology [9–11].
Previously DLBCL was subclassified based on cytomorphologic features into centroblastic, immunoblastic and anaplastic. Now it is classified based on variable clinical, morphologic, immunophenotypic, cytogenetic, and genetic features [6, 11]. Cell variables that may be important in predicting outcome are morphologic variants, bcl-2 expression, cell proliferation rate, p53 expression and mutation, and bcl-6 rearrangements [12, 13]. Studies have shown centroblastic lymphoma to be associated with a better prognosis than immunoblastic and bcl-2 is one of the strongest unfavourable prognostic factors which appear to confer resistance to chemotherapy [12].
CD5, T-cell marker expression can occur in DLBCL and may have prognostic significance. CD5+ DLBCL represents 5% to 10% of DLBCL, often associated with a more aggressive clinical course, poor outcome [14]. Aberrant co-expression of other T-cell antigens such as CD2, CD4, CD7, and CD8 has also been reported. CD20 and CD3 are shown to be most sensitive at assigning lineage. CD3 has been designated as the pan-T cell antigen, present on normal and neoplastic T lymphocyte. Recently few cases of large B-cell lymphoma with expression of CD3 and B-lineage markers have been reported. The proposed mechanisms are depression of genetic material, transformation of progenitor cell before its full divergence of B lymphocyte pathway, neoplastic expansion of normal B cells that express T-cell antigens [15]. There is morphologic, immunophenotypic diversity in DLBCL with few cases expressing T-cell markers. Expression of CD3 is rare and its significance, the underlying mechanism in such cases is still not understood. The present case showed that a subgroup of patients with initial early-stage DLBCL, favorable IPI, may show a risk of late relapse. And also highlights the possibility of oral lymphomas being an early manifestation of relapse.
Conclusion
Since advances in treatment modalities have improved the prognosis of this disease and many patients are long-term survivors, thus the risk of developing a second cancer, second lymphoma or a late relapse, principally in young patients is of growing concern. The symptoms of tooth mobility, teeth pain with preserved tooth vitality that is not related to other facts from clinical history should be dealt with caution. Intraoral lymphomas mimic inflammatory lesions, hence it is important for the dentist to be aware of various manifestations of NHL and an erroneous diagnosis may lead to diagnostic delay with poor prognosis.
[1]. Kolokotronis A, Konstantinou N, Christakis I, Papadimitriou P, Matiakis A, Zaraboukas T, Localized B-cell non- Hodgkin’s lymphoma of oral cavity and maxillofacial region: a clinical studyOral Surg Oral Med Oral Pathol Oral Radiol Endod 2005 99:303-10. [Google Scholar]
[2]. Ikeda T, Kanaya T, Matsuda A, Motohashi K, Tanaka H, Kohno N, Clinicopathologic study of non-Hodgkin lymphoma in sinonasal and hard palate regions in 15 Japanese casesORL J Otorhinolaryngol Relat Spec 2005 67(1):23-29. [Google Scholar]
[3]. Epstein JB, Epstein JD, Le ND, Gorsky M, Characteristics of oral and paraoral malignant lymphoma: a population-based review of 361 casesOral Surg Oral Med Oral Pathol Oral Radiol Endod 2001 92:519-25. [Google Scholar]
[4]. Van der Waal RI, Huigens PC, Van der VP, Van der Waal I, Characteristics of 40 primary extranodal non-Hodgkin’s lymphomas of the oral cavity in perspective of the new WHO classification and the International Prognostic IndexInt J Oral Maxillofac Surg 2005 34:391-95. [Google Scholar]
[5]. Gawęda A, Jach E, Wojciechowicz J, Sokołowska B, Tomaszewski T, Diffuse large B cell lymphoma of the oral cavity – case reportJ Pre-Clin Clin Res 2014 8(1):27-29. [Google Scholar]
[6]. Bhattacharyya I, Chehal HK, Cohen DM, Al-Quran SZ, Primary diffuse large B-cell lymphoma of the oral cavity: germinal center classificationHead Neck Pathol 2010 4(3):181-91. [Google Scholar]
[7]. Velez I, Hogge M, Primary maxillofacial large B-cell lymphoma in immunocompetent patients: report of 5 casesCase Reports in RadiologyVolume 2011Article ID 108023, 4 pages doi:10.1155/2011/108023 [Google Scholar]
[8]. Kalogeraki A, Tamiolakis D, Ligoxygkakis I, Sinatkas V, Papadakis M, Stathopoulos EN, Extra-nodal primary diffuse large B-cell lymphoma of the maxilla. Fine needle aspiration cytologyStomatologija 2013 15(2):58-60. [Google Scholar]
[9]. Larouche JF, Berger F, Chassagne-Clément C, Ffrench M, Callet-Bauchu E, Sebban C, Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: clinical characteristics and outcomeJ Clin Oncol 2010 28:2094-2100. [Google Scholar]
[10]. Cabanillas F, Velasquez WS, Hagemeister FB, McLaughlin P, Redman JR, Clinical, biologic, and histologic features of late relapses in diffuse large cell lymphomaBlood 1992 79:1024-28. [Google Scholar]
[11]. Martelli M, Ferreri AJM, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA, Diffuse large B-cell lymphomaCritical Reviews in Oncology/Hematology 2013 87:146-71. [Google Scholar]
[12]. Diagnostic Histopathology of Tumors, 3rd Edition, Volume-2, Churchill Livingstone Elsevier, 2007, Chapter 21, Tumors of the Lymphoreticular system, 1139-209 [Google Scholar]
[13]. Christine P. Hans, Dennis D. Weisenburger, Timothy C. Greiner, Randy D. Gascoyne, Jan Delabie, German Ott, Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarrayBlood 2004 103:275-82. [Google Scholar]
[14]. O’Malley D P, Auerbach A, Weiss L M, Practical applications in immunohistochemistry: Evaluation of diffuse large B-cell lymphoma and related large B lymphomadoi:10.5858/arpa.2014-0451-CP [Google Scholar]
[15]. Wang J, Chen C, Lau S, Raghavan R I, Rowsell E H, Said J, CD3- positive large B-cell lymphomaAm J Surg Pathol 2009 33:505-12. [Google Scholar]