Fixed Dose Combinations (FDCs) are defined by the World Health Organization (WHO) as combination of two or more active ingredients in a fixed ratio of doses [1]. FDCs must be shown to be safe and effective for the claimed indications and it cannot be assumed that benefits of the FDC outweigh its risks [2]. In last six years CDSCO has approved 303 FDCs [3]. This number is large when compared to the Essential Medicines List (EML) of WHO and national list of essential medicine [4,5]. As of now studies were mainly focusing on prescribing pattern of FDCs and their rationality [6,7]. Rationality status of many fixed dose combinations are marketed in India is not clear.
The aim of this study was to evaluate rationality of fixed dose combinations through pharmacokinetics (PK) and pharmacodynamics (PD) reasoning which were taken from Central Drugs Standard Control Organization (CDSCO) list.
Materials and Methods
Descriptive study was carried out at Department of Pharmacology, Pramukh Swami Medical College, Karamsad over a period of one year from April 2014 to August 2015. Ethical approval was taken from the Institutional Human Research Ethics Committee prior to study.
Inclusion Criteria
All FDCs, enlisted in CDSCO list from 2009 to 2014 were chosen for analysis.
Exclusion Criteria
Categories of FDCs like nutraceuticals, parenteral fluids used for hemodialysis & peritoneal dialysis, veterianary and cosmetics from Dermatology were excluded.
According to inclusion criteria, 264 FDCs were grouped according to systems like Cardiovascular, respiratory, GIT, allergy, Anti-infective, Pain/Musculoskeletal, Skin, Eye, Hematology, Endocrine and CNS. The following were recorded from each combination: 1) Year and system of FDC; 2) Dosage form; 3) Number of Active Pharmacological Ingredient (API); 4) Schedule of FDC; 5) The presence of the FDC and its ingredients in the WHO Essential Medicine List 2013 and National Essential Medicine List, India 2011; 6) PD and PK parameters of APIs of combination; 7) PK and PD interactions, and, 8) Safety parameters of ingredients in combination.
Rationality analysis for each combination was done using a rationality scoring scale which was developed according to WHO “Draft guidelines for registration of fixed-dose combination medicinal products” and the “Note for guidance on fixed-dose Proprietary Medicinal Products (CPMP), Europe” guidelines [2,8].
Rationality scoring score was developed for assessment of FDCs [Table/Fig-1]. Scientific evidence for the FDCs was assessed using accessible electronic and print sources of drug information like Medical journals, standard Pharmacology and Medicine text books, Pharmacopoeias, Formulary, Cochrane database, Pub Med etc [9–16]. Indian drug review (IDR), 2015 was used for drug scheduling [17].
Assessment of FDCs by Rationality Scoring Scale.
| Sr.no | Rationality Criteria | Yes | No |
|---|
| 1 | API from NLEM and WHO EML | All API (+1)At least one API (0.5) | 0 |
| 2 | Dose of API appropriate for intended use | +1 | 0 |
| 3 | Proportion of API appropriate for intended use | +1 | 0 |
| 4 | API should have different mechanism of action | +1 | 0 |
| 5 | PK and PD interaction | Favorable(+1)Not favorable(-1) | 0 |
| 6 | FDC facilitate dose reduction of API | +1 | 0 |
| 7 | FDC facilitate adverse drug reaction | +1 | 0 |
Maximum score= 9, Minimum score= 0
FDCs were graded as Irrational (0-<3), Semi-rational (3-<6), Rational (6-9).
Results
Out of 264 combinations, maximum numbers of FDCs 112 (42.42%) were approved in 2010, followed by 87 (32.95%) in 2009 [Table/Fig-2]. Maximum combinations 51 (19.31%) were from cardiovascular system followed by 46 (17.42%), 37 (14.01%), 25 (9.46%), 22 (8.33%), 20 (7.57%) combinations from Pain/Musculoskeletal, Anti-infective, Endocrine, Central Nervous System (CNS) and Respiratory System (RS) respectively which were approved by CDSCO from 2009 to 2014. Lowest number of combinations (5, 1.89%) was approved from hematological system [Table/Fig-2].
Year & system wise distribution of fixed dose combinations.
| System | Year |
|---|
| 2009n(%) | 2010n(%) | 2011n(%) | 2012n(%) | 2013n(%) | 2014n(%) | Totaln(%) |
|---|
| GIT (17) | 6 | 7 | 2 | 0 | 0 | 2 | 20 |
| (35.29%) | (41.17%) | (11.76%) | (0%) | (0%) | (11.76%) | (7.57%) |
| CVS (51) | 13 | 19 | 13 | 0 | 3 | 3 | 22 |
| (25.49%) | (37.25%) | (25.49%) | (0%) | (5.88%) | (5.88%) | (8.33%) |
| Haematological (5) | 0 | 0 | 4 | 0 | 1 | 0 | 17 |
| (0%) | (0%) | (80%) | (0%) | (20%) | (0%) | (6.43%) |
| RS (20) | 12 | 3 | 3 | 1 | 0 | 1 | 46 |
| (60%) | (15%) | (15%) | (5%) | (0%) | (5%) | (17.42%) |
| CNS (22) | 6 | 12 | 3 | 0 | 0 | 1 | 37 |
| (27.27%) | (54.54%) | (13.63%) | (0%) | (0%) | (4.54%) | (14.01%) |
| Pain/Musculoskeletal (46) | 13 | 20 | 9 | 3 | 1 | 0 | 5 |
| (28.26%) | (43.47%) | (19.56%) | (6.52%) | (2.17%) | (0%) | (1.89%) |
| Endocrine (25) | 5 | 11 | 3 | 0 | 2 | 4 | 25 |
| (20%) | (44%) | (12%) | (0%) | (8%) | (16%) | (9.46%) |
| Anti-infective (37) | 6 | 13 | 9 | 3 | 1 | 5 | 11 |
| (16.21%) | (35.13%) | (24.32%) | (8.10%) | (2.70%) | (13.51%) | (4.16%) |
| Eye (11) | 1 | 2 | 6 | 0 | 2 | 0 | 7 |
| (9.09%) | (18.18%) | (54.54%) | (0%) | (18.18%) | (0%) | (2.65%) |
| Skin (17) | 11 | 3 | 1 | 0 | 0 | 2 | 22 |
| (64.70%) | (17.64%) | (5.88%) | (0%) | (0%) | (11.76%) | (8.33%) |
| Allergy (7) | 1 | 4 | 2 | 0 | 0 | 0 | 17 |
| (14.28%) | (57.14%) | (28.57%) | (0%) | (0%) | (0%) | (6.43%) |
| Miscellaneous (6) | 0 | 6 | 0 | 0 | 0 | 0 | 6 |
| (0%) | (100%) | (0%) | (0%) | (0%) | (0%) | (2.27%) |
| Total n(%) | 87 | 112 | 61 | 9 | 11 | 23 | 264 |
| (32.95%) | (42.42%) | (23.10%) | (3.40%) | (4.16) | (8.71%) | |
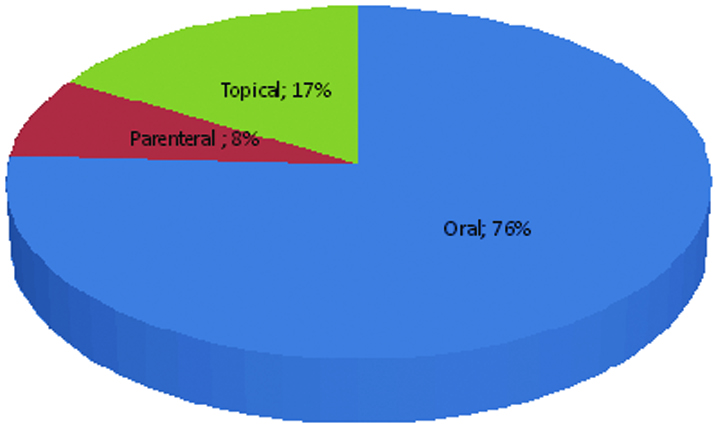
There were 200 (75.75%) combinations in oral dosage forms followed by 44 (16.66%) topical and 20 (7.57%) combinations in parenteral dosage forms [Table/Fig-3].
Distribution of FDCs according to dosage forms.
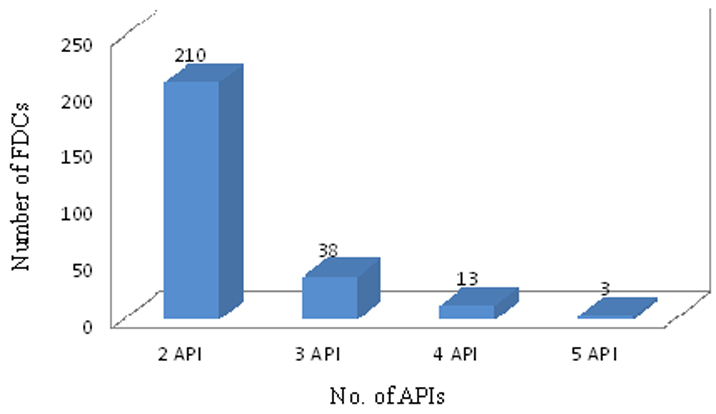
There were 210 (79.54%) of combinations having two active pharmacological ingredients (API) whereas only 3 (1.13%) combinations having 5 API [Table/Fig-4]. Out of total 264 combinations from CDSCO only 16 fixed dose combinations were from NLEM 2011, and 19, combinations from WHO EML 2013. There were 47 FDCs having all APIs included in the NLEM, whereas, 52 FDCs with all APIs included in WHO essential medicine list. Total of 115 combinations had at least one API enlisted in NLEM while 98 FDCs with at least one API in WHO list. It was found that there was not a single API from NLEM and WHO essential medicine list in 99 and 111 combinations respectively. Rest of the combinations was having 3 and 4 APIs [Table/Fig-5].
Number of Active Pharmacological Ingredients (API) in FDCs.
Distribution of FDCs according to number of API included in essential medicine list.
| System | No. of API included in EML |
|---|
| 11th NLEM [12] | 18th WHO Essential list [11] |
|---|
| All API(n) | At Least One API(n) | None API(n) | All API(n) | At Least One API(n) | None API(n) |
|---|
| GIT (17) | 1 | 8 | 8 | 0 | 4 | 13 |
| CVS (51) | 9 | 27 | 15 | 6 | 27 | 18 |
| Hematological (5) | 5 | 0 | 0 | 5 | 0 | 0 |
| RS (20) | 1 | 6 | 13 | 1 | 3 | 16 |
| CNS (22) | 2 | 10 | 7 | 2 | 10 | 7 |
| Pain /Musculoskeletal (46) | 3 | 22 | 21 | 1 | 22 | 23 |
| Endocrine (25) | 5 | 19 | 1 | 6 | 18 | 1 |
| Anti-infective (37) | 15 | 14 | 8 | 26 | 5 | 6 |
| Eye (11) | 1 | 2 | 8 | 1 | 1 | 9 |
| Skin (17) | 3 | 5 | 9 | 2 | 6 | 9 |
| Allergy (7) | 0 | 1 | 6 | 0 | 1 | 6 |
| Miscellaneous (6) | 2 | 1 | 3 | 2 | 1 | 3 |
| Total (n) | 47 | 115 | 99 | 52 | 98 | 111 |
Increased adverse drug reactions due to its active pharmacological ingredients were found in 123(46.59%) FDCs. Out of 123 combinations, maximum 24(47.05%) from cardivascular system followed by 22(47.82%) combinations from pain/musculoskeletal and 15(40.54%) FDC from anti-infective [Table/Fig-6]. Dose reduction in active pharmacological ingredients was seen in 58(21.96%) FDCs. Maximum dose reduction was seen with 22(43.13%) combinations in cardiovascular system followed by 19(51.35%) in anti-infective. No dose reduction was seen in any FDC on skin, eye, central nervous system, allergy, gastrointestinal and pain/musculoskeletal system [Table/Fig-6].
Assessment of increased chances of adverse drug reactions & dose reduction due to combinations.
| System | No. of FDC With Increased Chances of ADRs (%) | No. of FDCs With Dose Reduction (%) |
|---|
| GIT (17) | 8 (47.05%) | 0 (0%) |
| CVS (51) | 24 (47.05%) | 22 (43.13%) |
| Hematological (5) | 3 (60%) | 2 (40%) |
| RS (20) | 4 (20%) | 8 (40%) |
| CNS (22) | 13 (59.09%) | 0 (0%) |
| Pain /Musculoskeletal (46) | 22 (47.82%) | 0 (0%) |
| Endocrine (25) | 11 (44%) | 5 (20%) |
| Anti-infective (37) | 15 (40.54%) | 19 (51.35%) |
| Eye (11) | 6 (54.54%) | 0 (0%) |
| Skin (17) | 11 (64.70%) | 0 (0%) |
| Allergy (7) | 3 (42.85%) | 0 (0%) |
| Miscellaneous (6) | 3 (50%) | 2 (33.33%) |
| Total n (%) | 123 (46.59%) | 58 (21.96%) |
According to software Medscape, there was not a single PK and PD interaction found in between ingredients of any of the combinations but we found that there was a possibility of having PK interaction with 10 (3.78%) combinations and PD interactions with 73 (27.65%) combinations on the basis of standard textbooks and references which were taken in to consideration of rationality scoring scale.
Finally, according to rationality scoring scale, maximum combinations 137 (51.89%) were found to be irrational (1.68 ± 0.81) followed by 75 (28.40%) combinations were semirational (4.40 ± 0.56) and 52 (19.69%) was rational (6.65 ± 0.83) [Table/Fig-7]. Highest number of combinations 37 (80.43%) were evaluated as irrational in combinations for pain/musculoskeletal system followed by 21 (41.17%) combinations for cardiovascular system. Out of gastrointestinal, pain/musculoskeletal, eye, allergy and skin category, none of the combinations was found to be rational [Table/Fig-7].
Rationality Scoring scale of Fixed Dose Combinations.
| System | Scoring |
|---|
| 0-<3(Irrational)No of FDC(%) | 3-<6(Semi-rational)No of FDC(%) | 6-9(Rational)No of FDC(%) |
|---|
| GIT (17) | 15 (88.23%) | 2 (11.76%) | 0 (0%) |
| CVS (51) | 21 (41.17%) | 15 (29.41%) | 15 (29.41%) |
| Hematological (5) | 0 (0%) | 3 (60%) | 2 (40%) |
| RS (20) | 10 (50%) | 5 (25%) | 5 (25%) |
| CNS (22) | 15 (68.18%) | 5 (22.72%) | 2 (9.09%) |
| Pain /Musculoskeletal (46) | 37 (80.43%) | 9 (19.56%) | 0 (0%) |
| Endocrine (25) | 11 (44%) | 8 (32%) | 7 (28%) |
| Anti-infective (37) | 8 (21.62%) | 10 (27.02%) | 19 (51.35%) |
| Eye (11) | 5 (45.45%) | 6 (54.54%) | 0 (0%) |
| Skin (17) | 12 (70.58%) | 5 (29.41%) | 0 (0%) |
| Allergy (7) | 0 (0%) | 7 (100%) | 0 (0%) |
| Miscellaneous (6) | 4 (66.66%) | 0 (0%) | 2 (33.33%) |
| Total (264) | 137 (51.89%) | 75 (28.40%) | 52 (19.69%) |
| Mean ± SD | 1.68 ± 0.81 | 4.40 ± 0.56 | 6.65 ± 0.83 |
Discussion
Present study was done on assessment of FDCs with special inference to their rationality. Most of the studies on fixed dose combinations were related to prescribing pattern of combinations in different set up and diseases.
Out of total 264 FDCs taken for rationality, maximum combinations were in oral dosage form followed by 44 (16.66%) in topical and rest 20 (7.57%) in parenteral dosage form. Similar result was seen in Balat et al., combinations were most commonly prescribed by oral route (92.7%) followed by topical (5.9%) and parenteral (1.4%) routes (p < 0.001) [18]. According to Shah et al., all cardiovascular fixed dose combinations have oral dosage form [19].
There were 16 FDCs which were included in NLEM while 19 FDCs in WHO EML. Singh et al., analysed 225 prescriptions, out of which only 45 (20%) contained FDCs as recommended by WHO list of FDCs [20]. According to Dahiya A et al., approximately 20% of the FDCs (19.7%) were present in the WHO EML 2011 and 18.9% FDCs were present in the NEML, India 2011 [21].
In our study 123 (46.59%) FDCs had chances of increased adverse drug reactions due to their active pharmacological ingredients. Singh et al., also showed in his study out of 270 combinations, 150 (56.81%) fixed dose combinations were adding up adverse drug reactions. More than half combinations showed adverse drug reactions in studies by Rayasam et al., and McGettigan et al., [22,23]. One of the examples from present study is combination of (paracetamol 500 mg + nimesulide 100) mg in oral dosage form approved by CDSCO. As per WHO there is no need for addition of paracetamol in combination with nimesulide because this combination adds liver toxicity. In addition nimesulide has more antipyretic and analgesic property than paracetamol so it does not gain any advantage over paracetamol [24].
According to McGettigan et al., Thicolchicoside had been withdrawn by FDA from American and European market due to risk of aneuploidy. In this study we have observed that there are still 11 combinations in CDSCO list with thiocolchicoside and marketed n India [23].
With reference to anti-infective system, we observed good numbers of rational combinations in Anti-infective like (amoxicillin + Potassium clavulanate), (Imipenem + Cilastatin), (Cefixime + Ofloxacin). In addition all combinations of antiretroviral therapy also revealed rational. Similar finding was seen in study by Mehta et al., [25]. Combinations of cephalosporin with sulbactum/ tazobactum/Potassium clavulanate were found to be irrational. Reason behind it as beta lactamase inhibitors prevent destruction of beta lactam ring in penicillin group of antibiotics and thus widen the antibacterial spectrum of latter. However, these are not effective against the extended spectrum cephalosporins such as ceftazidime, ceftriaxone, cefotaxime [26]. In anti-infective system, we revealed 8 (21.62%) rational combinations and 19 (51.35%) irrational FDCs. Shah et al., developed the total score ranging from 1 to 12 and score ≥7 was considered to be rational and which was found out of 108 antimicrobial combinations, 21 (19%) were rational and 87 (81%) were irrational. Difference was seen from our result due to combinations from CDSCO list whereas the said study considered Indian Drug Review 2013.
The most pressing concern with irrational FDCs was related to (Eberconazole + Mometasone Furate) cream, (Beclomethasone Dipropionate + Neomycin sulphate) cream, (Halobetasol + Fusidic acid) cream, (Gatifloxacin + Loteprednol Etabonate + Benzalkonium Chloride) eye drop etc. This is because combination of antibacterial with corticosteroid which decreases the immunity and increases the susceptibility to infections, in addition patients never have bacterial, fungal and viral infection together that is why do not require all API together.
Our study considered all combinations with serratiopeptidase as irrational. Reason behind introducing the same claimed that enzyme it could promote rapid resolution of inflammation. But we could not find any evidence in published literature such as standard books or peer reviewed scientific journals supporting this claim [27].
According to scoring out of 264 combinations 52 (19.69%) FDCs scored in between (6-9), followed by 75 (28.04%) between (3-<6) and rest 137 (51.89%) combinations in between (0-<3). More than half combinations came to irrational (1.68 ± 0.81) in our observation from CDSCO list. Majority of them are from pain / musculoskeletal, skin, eye and GIT system. Dahiya et al., also revealed on the scale of rationality score 0-10 of which majority of the FDCs had rationality scores in low to intermediate range. A score of 6 was assigned to 26.4% of FDCs, 22.4% FDCs had a score of 3, 15.6% FDCs had a score of 2 and 15% FDCs had a score of 1. No FDC was assigned a score of 0.
Hence our study showed that major group of the combinations approved in last six years were found to be semi-rational and irrational. It is important to carry out detailed study in this area to establish the fact and increase rationality of the combinations.
Limitation
Analysis of combinations with reference to brand and generic separation was not done. Only FDCs approved in CDSCO list by DCGI were taken into study, other combinations available in the market were not analysed. One of the criteria to assess rationality scoring is cost analysis which was not done in this study because of huge variability of cost from different pharmaceutical companies.
Conclusion
It can be concluded that a large number of combinations are available in Indian market possessing semi-rational and irrational status for treatment various conditions. It is unethical to expose the patients to medicines with unproven efficacy safety. This calls for detailed study in this area to establish the fact and increase rationality of combinations.