Bladder cancer is one of the most prevalent malignancies of the genitourinary system, which comprises 4% of all malignancies [1]. Bladder cancer comprises approximately 2-3% of malignancies in women and is the ninth leading cause of death due to cancer in men [2]. In 25% of cases, bladder cancer occurs in muscle-invasive form and in 75% of cases in non muscle-invasive form [3]. Non-invasive bladder cancer is usually treated with transurethral resection (TUR) with or without intravesical therapies and has a good prognosis [4], while the radical cystectomy, with or without chemotherapy or radiotherapy, should be done for invasive tumours [5]. Accurate preoperative staging of the tumour is the most important factor in correct management and appropriate selection of treatment. Inaccurate staging of tumours is a serious problem in the bladder cancer, so it is essential to use acceptable imaging modalities [6–8]. Abdominal sonography is not capable of detecting location of small lymph nodes and it is very difficult to see tumour in obese patients with low bladder volume [9]. Because of inability to detect the depth of tumour penetration into layers of bladder wall and involvement of adjacent organs, and high radiation dose and the need for injection of ionic contrast agent, computed tomography (CT) scan is not an ideal modality for staging [10].
High-contrast resolution, capability of imaging in different views and ability to change image contrast by means of various sequences have turned Magnetic Resonance Imaging (MRI) into the best imaging modality for bladder cancer staging [11]. Method of dynamic contrast enhanced (DCE)-MRI is a useful technique in which rapid enhancement of tumour by uptake of the contrast agent is compared to bladder wall, assisting in differentiating tumour from surrounding normal tissue. Buy et al., investigated MRI staging of bladder carcinoma and its correlation with pathologic findings and concluded that MRI showed detection of bladder tumour invasion into adjacent organs better than CT scan [12]. Studying method of DCE-MRI for staging of superficial bladder cancer, Scattoni et al., found that contrast enhancement increased the accuracy of the method in superficial cancer staging [13]. Having observed high temporal resolution of the method of DCE-MRI, Barentsz et al., concluded that use of this method to distinguishing between after-biopsy effects and malignancy was possible [14]. Tekes et al., evaluated the accuracy of dynamic MRI staging of bladder and observed that the accuracy of dynamic MRI staging was high in differentiating superficial tumours from invasive tumours and organ-confined tumours from non-organ-confined tumours of bladder [6]. Daneshmand et al., in a retrospective study on staging of invasive bladder cancer reported improvement of staging of tumours and lymph nodes by DCE-MRI [7]. In a study conducted by Ghafoori et al., MRI was reported as a reliable method with high accuracy in bladder cancer staging [15].
Materials and Methods
Forty-five patients with transitional cell carcinoma of bladder who were candidate for radical cystectomy (based on the results of sonography, CT scan, or urinalysis) referred to the Imaging Center of Shafa for DCE-MRI, from January 2013 to July 2014 with a urologist’s coordination. Patients with tumour diagnosed as T0 and TX stages and contraindications of MRI such as claustrophobia, pacemakers, auditory cochlear and implants and other metal objects in their body were excluded from the study. Patients’ participation in the study was voluntary. The study protocol and objectives were explained to all the patients and written informed consent was obtained from them.
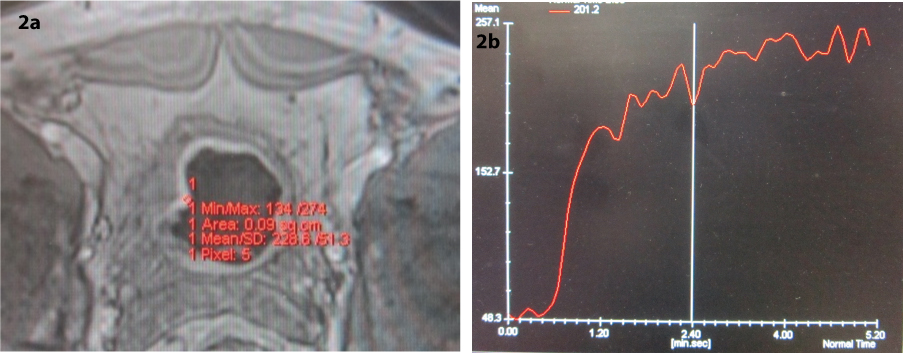
All the patients were imaged with an 18-channel 1.5 Tesla MRI scanner (MAGNETOM Avanto 18-channel SQ engine Siemens Healthcare, Erlangen, Germany) using a phased array coil. MRI protocol was as follows: axial, coronal and sagittal T1-weighted stir, axial T2-weighted fast spin echo, axial, coronal T1 fast spin echo, axial three-dimension volumetric interpolated breath-hold examination before and after intravenous injection of gadolinium (0.1 mmol/kg) [Table/Fig-1]. In order to prevent significant difference in arrival time of contrast agent and possible effect on the results, one person did imaging for all patients and manual injection of contrast agent in all the the patients lasted for 5-7 seconds with the rate of 3 cc/sec by the same needle. Starting injection of contrast agent was done simultaneously with data collection. Time-intensity curves were obtained from signal intensity values of the plotted ROIs. ROIs were drawn in the region of maximum enhancement and included five pixels [Table/Fig-2a&b].
Parameters of sequences for imaging of bladder and pelvic region.
| Parameter | Plane | TR(ms) | TE(ms) | NEX | Matrixsize | FOV(mm) | Slicethickness(mm) | Numberof Slices | Gap(mm) | Flipangle | Band with (Hz/Px) | TI (1) |
|---|
| Sequence |
|---|
| T1_3DVIBEa | Axial | 4.79 | 1.69 | 1 | 138*192 | 260 | 3.6 | 17 | 0.9 | 12 | 300 | - |
| T2WFSEb | Sagittal | 4240 | 80 | 2 | 256*205 | 300 | 5 | 18 | 1.2 | 150 | 211 | - |
| Axial | 2800 | 77 | 1 | 240*320 | 420 | 5 | 17 | 1 | 150 | 200 | - |
| T1WFSE | Axial | 500 | 11 | 1 | 320*192 | 336 | 5 | 18 | 1.2 | 180 | 178 | - |
| Coronal | 610 | 22 | 1 | 320*240 | 400 | 4 | 20 | 1.5 | 150 | 186 | - |
| T2W GREcTrue FISPd | Coronal | 4.15 | 2.08 | 1 | 256*230 | 410 | 4 | 18 | 1 | 47 | 528 | - |
| STIRe | Axial | 2800 | 32 | 1 | 256*240 | 320 | 5 | 17 | 1.2 | 150 | 191 | 160 |
| Coronal | 2800 | 32 | 2 | 320*240 | 380 | 5 | 18 | 1.2 | 150 | 191 | 160 |
| Sagittal | 2800 | 32 | 1 | 320*240 | 380 | 5 | 18 | 1.2 | 150 | 195 | 160 |
a volumetric interpolated breath-hold examination; b fast-spin-echo; c gradient recalled echo; d fast imaging with steady-state precession; e short tau inversion recovery
a) Axial contrast-enhanced 3D volumetric interpolated breath-hold examination magnetic resonance image in a T1 stage tumour with ROI; b) Time-intensity curve of the ROI.
A radiologist who was blind to pathology staging results reviewed MRI images. Tumour staging was done and compared with the pathologic staging. In all the patients, radical cystectomy was selected as the treatment. For staging, the following guideline was used:
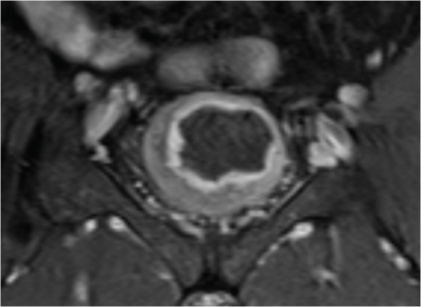
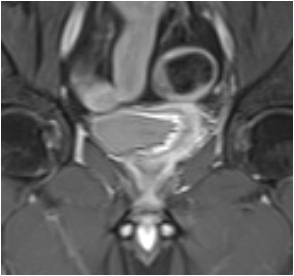
If the hypointense line at the base of tumour is preserved, it indicates an intact muscle layer and the tumour stage is T1 [Table/Fig-3] [17]; an irregular, shaggy inner margin of the hypointense line (muscle layer) indicates the stage T2a tumour; existence of a discontinuous hypointense line, without perivesical fat infiltration corresponds to the stage T2b [Table/Fig-4]; a lesion with an external irregular border with signal similar to tumour in the perivesical fat is defined as stage T3 [Table/Fig-5a&b]; and invasion to pelvic wall or adjacent organs indicates the stage T4 tumour [Table/Fig-6] [7,15,18]. As the depth of tumour invasion is highly effective on treatment planning, only T stage tumours were evaluated. Since, differentiating superficial tumours from invasive tumours is an important factor in appropriate management of cancer treatment, the accuracy of MRI in differentiating superficial tumours from invasive tumours was studied. For this purpose, we divided tumours into two groups: superficial (≤T1) and invasive (≥T2a) [6]. Patients with extravesical tumours have significantly higher recurrence rate, worse prognosis, and shorter survival than patients with organ-confined tumours [19], and differentiating between these two types of tumours is essential; thus, we also evaluated accuracy of MRI in differentiating between organ-confined tumour (≤T2b) and non-organ-confined (≥T3b).
Coronal contrast enhanced T1-weighted magnetic resonance image in 56-year-old man with transitional cell carcinoma of the bladder. Contrast enhanced images illustrate an intense enhancement of the tumour with uninterrupted detrusor muscle layer and intact perivesical fat, and mucosal involvement, staged as T1 tumour on MRI and pathology.
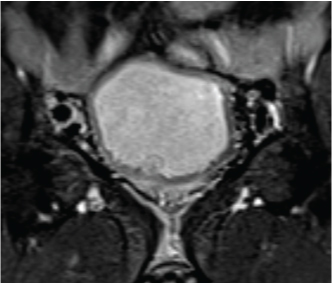
Coronal T1-weighted short tau inversion recovery image in 49-year-old man with transitional cell carcinoma of the bladder. Images show disrupted detrusor muscle without perivesical fat infiltration. These findings imply that tumour stage is T2b. The pathology stage was the same.
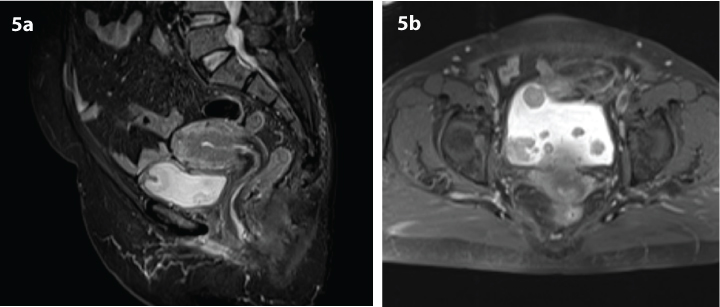
a) Sagittal T1-weighted short tau inversion recovery; b) Axial fat suppressed T1-weighted magnetic resonance images in 45-year-old woman with correctly staged T3b transitional cell carcinoma of the bladder. Images show deep invasion of the tumour to right bladder wall, bladder dome, and perivesical fat infiltration.
Coronal contrast enhanced T1-weighted magnetic resonance image in 50-year-old man with transitional cell carcinoma of the bladder. Images show invasion to the prostate gland. This was staged as T4a on MRI. The first transurethral resection staged this as T2b; but radical cystectomy confirmed a T4 tumour.
Ethics
This study protocol was approved the ethics committee of the university.
Statistical Analysis
The mean and variance were used to describe the quantitative data, and percentage and frequency were used for qualitative data. To assess the accuracy of DCE-MRI sensitivity, specificity, positive predictive value, negative predictive value, and positive and negative likelihood, the ratio between MRI and pathological findings was obtained; kappa agreement coefficient between MRI and pathology was calculated. Spearman’s correlation coefficient was calculated to examine the correlation between tumour stage and slope of time-intensity curve. Data were analysed by SPSS version 16 and the level of significance in all tests was considered p<0.001.
Results
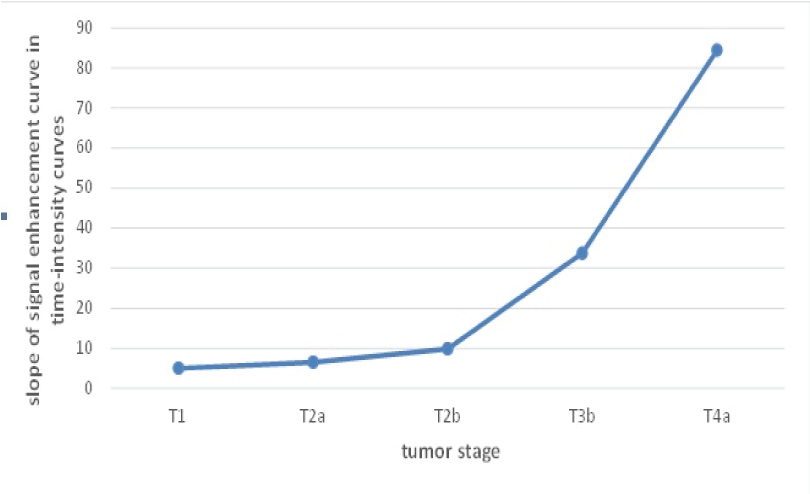
In this study, 43 male patients (95.55%) and two female patients (4.45%) were studied. Range of the patients’ ages was 43-63 years with mean of 52.07± 6.51 years. All tumours were transitional cell carcinoma in the histopathologic results. The most common stage seen in the MRI results was T3b (n: 19, 42%) [Table/Fig-7]. In the histopathologic findings, the most diagnosed stage in the patients was T3b (n: 15, 33%), as well [Table/Fig-7]. In addition three patients (6%) with stage T1, five patients (11%) with stage T2a, 10 patients (22%) with stage T2b and 8 patients (17%) with stage T4a were diagnosed. For the location of the tumour, the most common location of tumour were the left wall of the bladder, followed by posterior wall, anterior wall, base and dome of the bladder. Kappa agreement coefficient between MRI and pathology was obtained 0.7 (p<0.001). Totally 10 cases of mismatch between MRI and pathological staging were observed that according to [Table/Fig-7], in eight cases (80%) were overestimated and in two cases (20%) were underestimated. Detection rate of MRI was 0% in stage Ta, 100% in stage T1, 66.7% in stage T2, 86.7% in stage T3, and 100% in stage T4. Sensitivity and specificity of MRI (compared with histology as the gold standard) in differentiating superficial tumours (Ta, T1) from deep tumours (T2, T3, T4) were derived 0.97 and 1, respectively [Table/Fig-8]. Moreover, sensitivity and specificity of MRI in differentiating organ-confined tumours (Ta, T1, and T2) from non-organ-confined tumours (T2, T3, and T4) were obtained 0.94 and 0.77, respectively [Table/Fig-9]. Accuracy of MRI in differentiating superficial tumours (≤T1) from invasive tumours (≥ T2a), and organ-confined tumours (≤T2b) from non-organ-confined tumours (≥T3b) was 0.97 and 0.84, respectively. The overall accuracy of MRI in this study was 77.0 (p<0.001). Spearman’s correlation coefficient between signal enhancement slope of time-intensity curves and stage of tumours was obtained 0.88 (p<0.001) [Table/Fig-10].
Pathology and magnetic resonance imaging results.
| MRI | Total |
|---|
| T1 | T2a | T2b | T3b | T4a |
|---|
| Pathology | Ta | 1 | 0 | 0 | 0 | 0 | 1 |
| T1 | 1 | 0 | 0 | 0 | 0 | 1 |
| T2a | 1 | 5 | 0 | 5 | 0 | 11 |
| T2b | 0 | 0 | 9 | 1 | 0 | 10 |
| T3b | 0 | 0 | 1 | 13 | 1 | 15 |
| T4a | 0 | 0 | 0 | 0 | 7 | 7 |
| Total | 3 | 5 | 10 | 19 | 8 | 45 |
Diagnostic indices of magnetic resonance imaging versus histopathology in differentiation of superficial and deep tumours.
| Result | Point Estimate | Lower CI a | Upper CI |
|---|
| Sensitivity | 0.9767 | 0.9317 | 1.0218 |
| Specificity | 1 | 1 | 1 |
| Positive Predictive Value | 1 | 1 | 1 |
| Negative Predictive Value | 0.6667 | 0.1332 | 1.2001 |
| Positive Likelihood Ratio | infinity | - | - |
| Negative Likelihood Ratio | 0.0233 | 0.0034 | 0.1614 |
a Confidence interval.
Diagnostic indices of magnetic resonance imaging versus histopathology in differentiation of organ-confined and non-organ-confined tumours.
| Diagnostic Index | Point Estimate | Lower CI a | Upper CI |
|---|
| Sensitivity | 0.9444 | 0.8386 | 1.0503 |
| Specificity | 0.7778 | 0.6210 | 0.9346 |
| Positive Predictive Value | 0.7391 | 0.5597 | 0.9186 |
| Negative Predictive Value | 0.9546 | 0.8675 | 1.0416 |
| Positive Likelihood Ratio | 4.25 | 2.0801 | 8.6835 |
| Negative Likelihood Ratio | 0.0714 | 0.0105 | 0.4850 |
a Confidence interval.
Correlation between slopes of time-intensity curve with tumours stage.
Discussion
In our study, the most diagnosed MRI and pathologic stage was T3b. In other studies, the most commonly diagnosed stage was T1. This difference may be due to the fact that our patients’ referring was not early and the treatment started later [20,21]. According to the MRI findings and comparison of stages with pathologic findings, there was totally 10 mismatches between MRI findings and pathology, two (20%) of which were underestimated and eight (80%) were overestimated.
In a study by Tekes et al., consistent with our study, most cases of mismatches (32%) were overestimated (p<0.001) [6]. Buy et al., in a study with a 5.0 Tesla system without injection of contrast agent fewer of patients, as compared to our study, reported underestimation as 33% (p<0.001) [12]. In some studies, such as Scattoni et al., and Tekes et al., because of consecutive errors in determining stage of the bladder cancer, estimates were usually reported as overestimated [6,13].
Another reason for more overestimation than underestimation in our study was abnormal signals in perivesical fat in tumour location that were incorrectly identified as tumour invasion. For example, tumours were reported as T3b in five patients in stage T2b. Vascular hyperaemia adjacent to the tumour or inflammatory process that occurred in the tissues around bladder following TUR biopsy of tumours could cause these abnormal signals. In addition, chemical shift artifact at the bladder-perivesical fat junction may obscure the bladder wall or be regarded as pathological.
One case of underestimation occurred between T1 and T2a, because of underestimation of tumour invasion into hyposignal muscle layer of the bladder. Another case was between T3b and T2b; because of low signal to noise ratio of the image and slight movement of the patient, perivesical invasion remained hidden from sight of radiologist.
Accuracy of DCE-MRI in bladder cancer staging has been reported 52-93% [6,22] which could increase up to 73-100% with injection of contrast agent [6]. Accuracy of 77% in our study indicates a good correlation between MRI and pathology. In the study of Sohn et al., the accuracy of dynamic MRI staging was reported 83% that was slightly higher than accuracy obtained in our study, which could be due to greater number of samples in Sohn et al., study [23].
In study of Nguyen et al., use of a three Tesla scanner could be the reason for accuracy of 81% [24]. It has been demonstrated that tumours were understaged by TUR [18]. In addition, the weakness of MRI in detection of tumours in stage T3a (because of microscopic invasion of tumour) may also be the reason for lower accuracy obtained in the present study.
In the present study, kappa agreement coefficient was 0.7, and sensitivity and specificity in differentiating superficial tumours from invasive tumours were 97% and 100%, and in differentiating of organ-confined tumours from non-organ-confined tumours were 94% and 77%, respectively, which could be considered as acceptable and consistent with the results of Tekes et al. (95% sensitivity, 55% specificity) [6]. Takeuchi et al., achieved sensitivity and specificity of 80% and 92%, respectively, for MRI in differentiating organ-confined tumours from non-organ-confined tumours [20]. Abou-El-Ghar et al., observed that the accuracy of diffusion imaging in staging of organ-confined tumours was significantly higher than T2W imaging [25].
Gadolinium enhancement has a considerable effect on increasing the accuracy, sensitivity, and specificity [15,18]. By observing high temporal resolution of DCE-MRI, Barentsz et al., concluded that distinguishing between after-biopsy effects and malignancy was likely [14]. Kim et al., have concluded that dynamic gadolinium-enhanced MRI significantly increases the accuracy of staging of bladder cancer [14,17].
The present study showed that the MRI accuracy in differentiating superficial tumours from invasive tumours was 97% and in differentiating organ-confined tumours from non-organ-confined tumours was 84%, which is consistent with Tekes et al., study with regard to differentiating organ-confined tumours from non-organ-confined tumours (82% accuracy) [6].
A point that was considered in the present study rather than previous studies was the correlation of tumour stage with slope of time-intensity curves of tumour in dynamic images. Tumour vessels are not normal and tumour epithelial cells within the inner surface of tumour develop gaps. The blood leaking out from the distance between endothelial cells, permeability, and neovascularity of tumour increases abnormally and tumours with higher angiogenesis have higher risk of local recurrence. By DCE, the peak of the curve occurs in areas with uptake of contrast, which represents increased permeability, abnormal vascularity, and higher malignancy of tumour [26]. The positive correlation and significant relationship between tumour stage and signal enhancement slope of tumour time-intensity curves suggests that the peak time-intensity curves occur with greater slope as tumour stage increases. This relationship can be helpful in diagnosis of severity and progression of tumours, as the slope of the curves is steeper in the non-organ-confined tumours.
Conclusion
In this study, despite small differences between the results of the MRI and pathology, DCE-MRI was found to be an accurate modality for assessment of T staging, and its routine use in bladder cancer staging causes significant improvement of diagnostic accuracy of the staging and treatment planning and hence improvement of the prognosis of patients and their survival rates. Furthermore, the use of MRI systems with higher magnetic field and imaging techniques standardized with higher resolution could further enhance the accuracy of the method. Comparison of MRI data with CT-scan findings of patients is recommended in future studies. Further studies with larger sample size may also help to validate the results of this study.
a volumetric interpolated breath-hold examination; b fast-spin-echo; c gradient recalled echo; d fast imaging with steady-state precession; e short tau inversion recovery
a Confidence interval.
a Confidence interval.