Plan for postoperative pain is the hallmark of a good anaesthetic practice. Pain relief after cesarean delivery is especially important as the consequences of inadequate pain relief are borne not only by the mother but by the newborn as well, since a parturient who is experiencing pain finds it difficult to feed her newborn [1].
Opioids, which otherwise are the mainstay analgesics in the postoperative period are avoided in the parturient since almost all opioids find their way in the milk predisposing the neonate to their adverse effects [2]. So other modalities for pain relief are often selected. Now-a-days, multimodal approach to pain relief is recommended so that adverse effects of individual drugs can be reduced. Neuraxial blocks, peripheral Nerve blocks, NSAIDS and local anaesthetic infiltration of wound have all been used as part of multimodal approach [3].
Local wound infiltration is an attractive strategy since it is efficacious and side effects are minimal [4]. However, this modality is limited by the fact that duration of analgesia is provided only till the effects of local anaesthetic action lasts. Efforts are being made to prolong the duration of action of local anaesthetic skin infiltration and magnesium is one such agent which has been used for this purpose [5].
To study the adjuvant analgesic effects of subcutaneous infiltration of magnesium in parturients undergoing caesarean delivery under spinal anaesthesia.
Materials and Methods
The study was conducted at a tertiary level hospital in a randomized double blinded manner on a total of 60 parturients belonging to American Society of Anaesthesiologists (ASA) grade I or II, scheduled for cesarean delivery under spinal anaesthesia. Prior approval from institutional ethics committee was taken and an informed consent was obtained from all patients. Patients with a history of drug abuse, patients with psychiatric disease, morbidly obese patients, patients who were unable to comprehend Visual Analogue Scale (VAS), failed spinal anaesthesia and conversion to general anaesthesia, or patients with history of allergic reactions to local anaesthetics, opioids and/or magnesium were excluded from the study. Patients were enrolled in the study after a thorough pre-anaesthetic check up and routine investigations which included a Complete Haemogram, Coagulation profile and Random Blood sugar. All patients were shown and explained regarding the use of Visual Analogue Scale (VAS) in the postoperative period and informed that they can request an analgesic at any time after surgery if they feel pain.
After shifting the patients to the operation theatre, pre induction Heart rate (HR), Non Invasive Blood Pressure (NIBP), Respiratory Rate (RR), Oxygen Saturation (SpO2) and Electrocardiography (ECG) were recorded. These parameters were monitored throughout the procedure and recorded every 10 minutes. An intravenous access (IV) was achieved and normal saline infusion commenced. After preloading with 10ml/kg body weight of IV fluids, all patients were administered subarachnoid block in the left lateral position under all aseptic precautions using a 26 Gauge Quincke’s needle at L3-4/ L 4-5 vertebral level injecting 2.0 ml of 0.5% heavy bupivacaine. Surgery was allowed to proceed after complete sensory block was achieved at T8 dermatome as assessed by pinprick.
In case of partial/failed spinal anaesthesia, general anaesthesia was administered and the patient was excluded from the study. Intra-operative complications like hypotension, bradycardia, nausea/vomiting, etc were managed as per departmental policy in both the groups. After the closure of uterus and muscle layer but before closure of skin, the allocated drug as per random grouping based on coded sealed envelope technique was administered by local subcutaneous wound infiltration at the incision site, by the obstetrician who was blinded to the study drug administered. This time was labeled as ‘0’ and recording of parameters was started from. Group A patients were administered a Local subcutaneous wound infiltration of Injection (Inj) ropivacaine 0.75% 150 milligrams (mg) or 20 millilitres (ml) whereas, group B patients were administered a Local subcutaneous wound infiltration of Inj magnesium sulphate 750 mg (1.5 ml of 50% Inj Magnesium sulphate) added to Inj ropivacaine 0.75%(18.5 ml) making the total volume of injectate to 20 ml. After this, skin closure was done and patients shifted to Post anaesthesia care unit (PACU).
On arrival to PACU, patients were asked to rate the pain using VAS rulers having slide indicator and were asked to bring the slider on the scale on to the point that they feel represents their current state of pain with ‘0’ mark corresponding to no pain and ‘10’ mark representing worst imaginable pain. Patients were monitored for postoperative pain and any analgesic requirement for a period of 24 hours.
Any patient complaining of pain or reporting VAS ≥4 at any time was administered Inj tramadol 100 mg IV slowly over 2-3 minutes. If pain was not relieved after 30 minutes and patients still complained of pain, additional doses of Inj tramadol 50mg IV was given and this dose could be repeated every 30 minutes upto a total dose of 250 mg in 6 hourly and maximum of 400mg of Inj tramadol over 24 hours. Time of first rescue analgesic administration and total rescue analgesic consumed in 24 hours postoperatively was noted. Patients were also evaluated for any adverse effects, 24 hours postoperatively [Table/Fig-1].
Consort Diagram for patients entering the study.
In the PACU, the following parameters were observed and recorded.
Heart rate, blood pressure, respiratory rate, SpO2 every 10 minutes for 1 hour and then every half hourly for the next 2 hours followed by every hour till 24 hours.
Assessment of pain using visual analogue scale every hour for the initial 4 hours and every 4 hourly after that.
Time of first rescue analgesic administration in the postoperative period.
Total analgesic consumption in the 24 hour postoperative period.
Patients were also observed for any adverse effect like postoperative nausea or vomiting, Skin rash (redness or itching), hypotension (defined as blood pressure less that 15% of baseline values), sedation (as per Ramsay sedation scale), respiratory depression (defined as respiratory rate less than 10/minute), need for supplemental oxygen (saturation less than 93%), bradycardia (heart rate less than 60 beats/min), any redness or signs of inflammation at the skin incision site.
Analysis of Data
After completion of the study, observations obtained were tabulated and data was expressed as mean and 95% confidence interval of mean for continuous variables (height, weight, duration, age). Data was analysed using Statistical Package for the Social Sciences (SPSS) version 15 (SPSS Inc, Chicago, IL). Comparison of continuous data between groups was done using independent t-test and Mann Whitney test respectively for parametric and non- parametric data. Comparison of nominal data was done using Chi-square analysis. The p-value less than 0.05 were considered statistically significant between groups.
Sample size for the study was estimated by taking into consideration the results of previous studies by Eldaba et al., and Tauzin et al., using G star power software [10,11]. Tauzin et al., had found that total 24-hour postoperative supplemental tramadol consumption in Group receiving intravenous magnesium was 221 ± 64.1 mg and 134 ± 74.9 mg in Group which received local subcutaneous magnesium. However, this study was conducted on Prostatectomy patients whose pain profiles are different from patients undergoing cesarean section. Eldaba et al., performed study on patients undergoing cesarean section and the study had 40 patients in each group, which gave the study a power of 100 % [10]. Based on this, a sample size of 60 patients was chosen to detect 10% difference with 90% power and α of 0.05.
Results
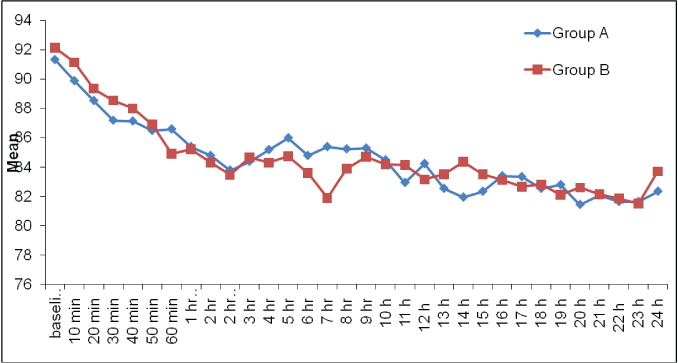
There was no significant difference among the two groups with respect to mean age, height, weight and gestational age. Majority of the patients had no previous history of cesarean section in either of the groups [Table/Fig-2]. Level of sensory block was similar in both the groups with no statistically significant difference. Postoperatively, the baseline heart rate were comparable between group A and group B (p =0.794). On intergroup comparison, there was no statistically significant difference in the mean heart rate among the two groups at any of the time intervals. On intragroup comparison, heart rate showed a falling trend in readings from baseline in both the groups [Table/Fig-3].
| Group A | Group B | p-value |
|---|
| Mean Age (Years) | 27.90±3.39 | 28.05±4.22 | 0.935 |
| Mean Weight (Kg) | 64.45±9.55 | 64.75±7.67 | 0.913 |
| Mean Height (cm) | 162.35±5.58 | 162.24±4.60 | 0.357 |
| Gestational Age (weeks) | 38.08±1.24 | 38.35±1.08 | 0.183 |
| Previous surgery (%age) | 40% | 35% | 0.744 |
Trends in postoperative heart rate.
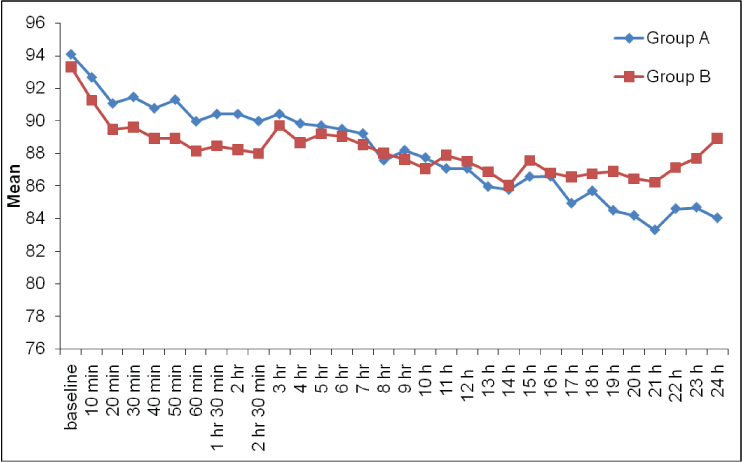
Postoperatively baseline mean arterial pressure was 94.07±8.98 mm Hg, and 93.30±5.52 mm Hg in group A and group B respectively. There was statistically no significant difference in mean arterial pressure among both the groups at any time interval [Table/Fig-4].
Trends in postoperative mean arterial pressure.
VAS at various time intervals was similar in both the groups with no statistically significant difference [Table/Fig-5]. The need for IV rescue analgesic for the first time was at 4.75±3.68 hours in group A and at 6.00+3.51 hours in group B [Table/Fig-6]. Thus, the need for first dose of rescue analgesia was earlier in group A as compared to group B but the difference was significantly not significant (p=0.279). However, the need for 2nd and 3rd doses of rescue analgesics was significantly later in group B and the difference was statistically significant with p-value of 0.034 and 0.031 respectively. The time for 4th rescue analgesic, however, was similar in both the groups with no statistically significant difference (p=0.227).
Mean VAS at various time intervals.
| Time (in hours) | Mean VAS Group A | Mean VAS group B | p-value |
|---|
| 0 | 2.87±0.73 | 2.48±0.45 | 0.487 |
| 1 | 2.77±0.83 | 2.75±0.68 | 0.565 |
| 2 | 2.95±0.67 | 2.87±0.26 | 0.380 |
| 3 | 2.86±0.88 | 3.20±0.67 | 0.482 |
| 4 | 2.99±0.75 | 2.64±0.80 | 0.346 |
| 8 | 2.97±0.67 | 2.72±0.17 | 0.275 |
| 12 | 3.37±0.59 | 3.14±0.34 | 0.176 |
| 16 | 3.99±0.78 | 3.78±0.27 | 0.267 |
| 20 | 3.55±0.77 | 3.36±0.35 | 0.341 |
| 24 | 4.61±0.80 | 4.14±0.45 | 0.546 |
Mean time to rescue analgesics.
| Mean timeto rescueanalgesics | Group A | Group B | p-value |
|---|
| Mean | S.D | Mean | S.D |
|---|
| 1st time | 4.75 | 3.68 | 6.00 | 3.51 | 0.279 |
| 2nd time | 9.70 | 5.12 | 13.85 | 5.43 | 0.034 |
| 3rd time | 14.31 | 3.94 | 18.33 | 2.42 | 0.031 |
| 4th time | 19.00 | 2.94 | 23.00 | - | 0.227 |
| 5th time | 21.40 | 1.82 | - | - | - |
The number of patients who were administered 2nd, 3rd and 4th doses of rescue analgesics was significantly greater in group A as compared to group B [Table/Fig-7]. None of the patients in group B needed more than 4 doses of rescue analgesia while in group A, 5 patients were administered a rescue analgesic for 5th time.
Total number of patients requiring rescue analgesic in each group.
| Group A | Group B | p-value |
|---|
| 1st time | 30 | 30 | - |
| 2nd time | 30 | 24 | 0.008 |
| 3rd time | 26 | 16 | 0.001 |
| 4th time | 21 | 11 | 0.001 |
| 5th time | 10 | 0 | 0.017 |
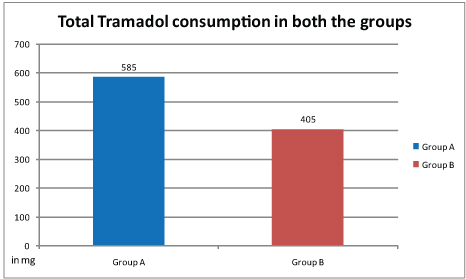
The cumulative analgesic requirement in group A was also greater in group A as compared to group B and the difference was statistically significant (p =0.01) [Table/Fig-8].
Cummulative IV Tramadol (in mg) consumption in 24 hours.
(p= 0.01).
None of the patients developed skin rash, respiratory depression or any signs of local wound inflammation [Table/Fig-9].
Incidence of adverse effects.
| Adverse effect(% age) | Group A | Group B | p-value |
|---|
| Skin rash | 0 | 0 | - |
| Nausea | 53.3 | 26 | 0.013 |
| Vomiting | 30.0 | 10 | 0.01 |
| Sedation | 16.66 | 10 | 0.672 |
| Hypotension | 16.66 | 20 | 0.872 |
| Pruritus | 38.66 | 30.66 | 0.776 |
| Respiratory depression | 0 | 0 | - |
| Need for supplemental oxygen | 0 | 0 | - |
| Signs of wound inflammation(redness, excessive swelling) | 0 | 0 | - |
Discussion
Concerns regarding opioid induced hyperalgesia and sensitization are growing and efforts are on to mitigate this opioid related adverse effect [12]. Recently, there is an interest in the use of NMDA antagonists like magnesium in postoperative pain relief. These agents have the potential to prevent central sensitization to peripheral nociceptive stimulation and also abolish hypersensitivity, if it is established [13].
The administration of intravenous magnesium in the peri-operative period, however, is fraught with risk, as it may potentiate neuromuscular blockade after administration of neuromuscular blocking drugs [14], increase sedation [15] and contribute to serious cardiac morbidity [16]. These adverse effects have brought attention towards subcutaneous administration of magnesium as an adjunct to the local anaesthetic agents [10].
The dose of ropivacaine used in our study is as per the recommended dosage guidelines and is well within the safety limits [17]. The dose of magnesium co-relates with the dose used by Tauzin et al., who used 750 mg of magnesium in 0.25% bupivacaine to a total volume of 20 ml [11]. Larger doses than the dose administered by us, have been safely used in parturients in earlier studies [15].
The mean heart rate and mean blood pressure did not change significantly from baseline suggesting there are no adverse cardiovascular adverse effects of a small dose of magnesium when used for subcutaneous infiltration. Our results are similar to those of Donadi et al., who also observed no significant change in blood pressure on using magnesium [18].
VAS in both the groups was similar in both the groups at various time intervals whereas, the total supplemental analgesic consumption was higher in group B. This was expected, as patients were administered supplemental IV analgesics whenever, patients reported VAS more than 3. For this reason, supplemental analgesic consumption may give a better idea regarding the effectiveness of adjuvant added to the local anaesthetic infiltration. The need for first dose of supplemental analgesic was later in the group B as compared to group A though the difference was statistically not significant. Afterwards, the second and third supplemental doses of analgesics were consumed much later in group B as compared to group A, though there was no significant difference in the timing of 4th dose. Group A received five doses of supplemental analgesics as compared to four doses in the group B. This could explain the statistically similar timings of 4th supplemental dose of analgesic in the group B. Our results are similar to Eldaba et al., who used continuous wound infiltration of bupivacaine along with magnesium sulphate in patients undergoing caesarean section and reported an effective analgesia and reduced postoperative Patient controlled analgesia (PCA) requirements as compared to continuous wound infiltration with local anaesthetic only or placebo [10]. The total analgesic consumption in our study in the initial 24 hours was also significantly reduced in group B as compared to group A. Lee et al., also reported reduced opioid consumption in patients who received wound infiltration with magnesium [12].
None of the patients suffered from bradycardia, hypoxaemia, respiratory depression, skin rash or incision site excessive redness nor was there any evidence of infection. The incidence of sedation and pruritis was similar in both groups with no statistically significant difference. The incidence of nausea and vomiting was infact lower in group B as compared to group A. This could be explained by the lesser use of rescue analgesic agent in group B since Inj tramadol itself is associated with increased incidence of nausea and vomiting [19]. Eldaba et al., also did not observe any significant adverse effects with the subcutaneous infiltration of bupivacaine and magnesium mixture [10].
The result of our study brings out some interesting findings. The study suggests that local infiltration of local anaesthetic agent alone or in conjunction with magnesium is safe. The addition of magnesium to local anaesthetics potentiates the effect of local anaesthetics and reduces the postoperative opioid requirement. Our results are similar to studies by Eldaba et al., and Dunadi et al., which reported similar findings [10,18]. Thus, while the potential adverse effects of IV magnesium mentioned above are avoided, still the benefits accrued by its adjunct analgesic effect can be availed. Thus, subcutaneous infiltration in conjunction with local anaesthetic agents holds great promise.
Limitations
Our study however, has certain limitations. The dose of magnesium sulphate was arbitrarily selected and blood levels of ropivacaine and magnesium were not estimated. Secondly, patients were observed only for 24 hours, the study would have been more elaborative, if the study period was longer and patients were followed up at an interval of few months to look for chronic pain. Thirdly, all of our patients belonged to ASA grade I and II with no severe underlying disease, therefore the results of the present study should not be generalized to all the patients. More studies are needed in future to delineate the role of magnesium in the multimodal pain management strategies.
Conclusion
The results of our study indicate that subcutaneous infiltration of magnesium reduces postoperative analgesic requirements after Cesarean delivery and is not associated with any significant adverse effects.