Antibacterial Activity and Fluoride Release of Glass-Ionomer Cement, Compomer and Zirconia Reinforced Glass-Ionomer Cement
Sonia Tiwari1, Mallikarjuna Kenchappa2, Deepak Bhayya3, Shilpi Gupta4, Sudhanshu Saxena5, Saurabh Satyarth6, Aishwarya Singh7, Manoj Gupta8
1 Post Graduate Student, Department of Pedodontics and Preventive Dentistry, Hitkarini Dental College and Hospital, Jabalpur, Madhya Pradesh, India.
2 Professor, Department of Pedodontics and Preventive Dentistry, College of Dental Sciences, Davangere, Karnataka, India.
3 Professor and Head, Department of Pedodontics and Preventive Dentistry, Hitkarini Dental College and Hospital, Jabalpur, Madhya Pradesh, India.
4 Reader, Department of Pedodontics and Preventive Dentistry, Hitkarini Dental College and Hospital, Jabalpur, Madhya Pradesh, India.
5 Reader, Department of Public Health Dentistry, Hitkarini Dental College and Hospital, Jabalpur, Madhya Pradesh, India.
6 Post Graduate Student, Department of Pedodontics and Preventive Dentistry, Hitkarini Dental College and Hospital, Jabalpur, Madhya Pradesh, India.
7 Senior lecturer, Department of Public Health Dentistry, People’s College of Dental Sciences & Research Center, Bhopal, Madhya Pradesh, India.
8 Post Graduate Student, Department of Public Health Dentistry, People’s College of Dental Sciences & Research Center, Bhopal, Madhya Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sonia Tiwari, Post Graduate Student, Department of Pedodontics and Preventive Dentistry, Hitkarini Dental College and Hospital, Jabalpur-482005, Madhya Pradesh, India.
E-mail: soniatiwari2006@gmail.com
Introduction
The cariostatic property of glass ionomer cement (GIC) stems from its ability to release fluoride into the oral environment. Recently, zirconia reinforced GIC has been launched which promises the protective benefits of glass ionomer while completely eliminating the hazard of mercury.
Aim
To evaluate invitro antibacterial activity and fluoride release from two conventional glass ionomer cements (GC II and GC IX), compomer (Compoglass) and a zirconia reinforced glass ionomer cement (Zirconomer).
Materials and Methods
The antibacterial activity of the cement specimens was evaluated against Streptococcus mutans using the agar inhibition test. Zone of inhibition on Mueller-Hinton agar plates was measured after 48 hours. The fluoride release from the cement specimens in ppm were measured at day 1, 7, 14 and 21 using a fluoride ion selective electrode.
Data was analysed using one-way and two-way analysis of variance (ANOVA) followed by LSD post-hoc test. A p-value <0.05 was considered statistically significant.
Results
Statistically significant largest zone of inhibition was observed with Zirconomer. Also, significant differences were seen in fluoride release of different materials. At all the time intervals maximum fluoride release was observed with Zirconomer and minimum with Compoglass.
Conclusion
This invitro investigation has revealed that zirconia reinforced GIC (Zirconomer) had maximum antibacterial activity against S.mutans and fluoride release.
Dental caries, Invitro technique, Streptococcus mutans, Zirconium oxide
Introduction
Restoring carious teeth is one of the major treatment needs of young children [1]. Amalgam is the most commonly used restorative material in dentistry over the last 150 years. However, hazards of mercury from amalgam restorations on human health and environment have impelled the development of new restorative materials [2]. Also, secondary caries has been quoted as one of the most common cause of restoration failures [3]. Microleakage can lead to accumulation of bacteria underneath the restoration [4]. Streptoccous mutans is primarily associated bacterium in dental caries [5]. Available literature reveals that approximately 60% of total replacements of restorations are due to secondary caries. In the process of replacing existing restoration, the size of restoration changes considerably [3]. The ability of dental restorative materials to inhibit recurrent caries is an important clinical property [6]. Fluoride release from restorative materials is important because of probable caries inhibitory effect [7]. There are several fluoride containing dental restorative materials available in the market including glass ionomer cements, resin modified glass ionomers, polyacid modified resins (compomers), giomers and resin composites [8].
After the invention in 1972 by Wilson and Kent, Glass Ionomer Cements (GICs) were recommended in 1977 as restorative materials in deciduous dentition because of their fluoride release ability and adhesive nature to dental hard tissues [1,9]. Relative lack of strength and low resistance to abrasion and wear are the major limitations of conventional GICs in clinical practice [1]. To overcome this, several modifications in conventional GICs have been done. GIC type IX is a newer more viscous, aesthetic reinforced GIC especially developed for atraumatic restorative treatment [10].
Extensive research has been conducted on conventional and resin modified GIC to evaluate their success as restorative materials for deciduous teeth. But mostly results have been unsatisfactory, particularly in proximal cavities where the restoration is comparatively unsupported [11]. The performance of GIC is better in single-surface restorations compared to multi-surface restorations due to brittle nature of material which necessitates support of the surrounding tooth structure. GIC with high powder: liquid ratio such as Ketac Molar (3M-ESPE, Seefeld, Germany), Fuji IX GP (GC America Inc., Alsip, Illinois, USA) and Chemflex (Dentsply, York, Pennsylvania, USA) were marketed in mid-1990s. Clinical studies using these relatively stronger materials have shown results better than earlier type 2 GICs in proximal cavities of deciduous teeth [11].
Compomers were introduced as a new class of restorative materials which offered the combined benefits of composite resins (“comp” in the name) and GIC (“omer”). Compomer encompasses all the key constituents of composites and GIC except water. Lack of water prevents the setting of compomer in its container. When visible curing light is applied, setting of compomer takes place by bonding of the molecules of Urethane Dimethacrylate (UDMA) and Butane-Tetra Carboxyl Acid (TCB) in a three-dimensional net. The carboxylic acid monomer (TCB) undergoes an equilibration reaction with the glass silicate. The GIC acid-base reaction occurs after exposure to moisture and results in a partially ionic structure within the polymeric matrix [3].
Recently, zirconia reinforced GIC has been launched by Shofu Inc., Kyoto, Japan. According to manufacturer it possesses the strength and durability of amalgam with the protective benefits of glass ionomer while completely eliminating the hazard of mercury [12]. Zirconia or zirconium oxide has been used as indirect restorative material since 1998. The strength of zirconia allows it to be used for crown and bridge restoration in all areas of mouth [13].
Release of fluoride from various reformations of GICs is a substantial contributor for their antibacterial property [5]. Fluoride release from restorative materials during short and long time period depends upon several aspects such as nature and amount of fluoride integrated, matrices of materials and setting reactions [14].
Aim
The present study was aimed to evaluate invitro antibacterial activity and fluoride release from two conventional glass ionomer cements (GC II and GC IX), compomer (Compoglass) and zirconia reinforced glass ionomer cement (Zirconomer).
Materials and Methods
The present invitro study was carried out in the Department of Pedodontics and Preventive Dentistry, Hitkarini Dental College and Hospital, Jabalpur, Madhya Pradesh in collaboration with Excellent Bio Research Solutions Private Limited (Daksh laboratories), Jabalpur, Madhya Pradesh. The study was carried out over a period of two months. As this was an invitro investigation, ethical clearance was not obtained.
The study was performed in two parts: (1) evaluation of fluoride release among four different cements at different time interval; and (2) antibacterial activity against Streptococcus mutans at 48 hours.
Materials tested were:
Group 1: Zirconia Reinforced Glass Ionomer Cement (Shofu Inc., Kyoto, Japan): 20 specimens,
Group 2: Glass Ionomer Cements II (GC corporation, Tokyo, Japan): 20 specimens,
Group 3: Glass Ionomer Cements IX (GC corporation, Tokyo, Japan): 20 specimens,
Group 4: Compomer (Compoglass, Ivoclar Vivadent Inc, Newyork, USA):20 specimens.
Preparation of Specimens
Twenty specimens of each experimental group of dimensions 5mm and 8mm depth were prepared using cylindrical shaped teflon moulds. Specimens for group 1, 2 and 3 were prepared by hand mixing with the help of paper mixing pad and agate spatula according to the manufacturer’s instruction and packed into the moulds. Each specimen was covered by a mylar strip and glass cover slips and allowed to set at room temperature 37°C. The light cure samples (compomer), after packing of material into moulds were cured for 40 seconds from top and bottom by irradiation with a halogen bulb emitting blue light (470 nm wavelength and 75 watts nominal intensity). An additional 20 seconds light curing was done in the middle of sample from both sides.
Estimation of Fluoride Release
Set specimens after being removed from the moulds were suspended in 1ml of deionized water in plastic vials and stored at 37°C and agitated from time to time. After 24 hours, careful removal of specimens from the solution, drying and transferring into a new vial containing fresh distilled water at 37°C was performed and same procedure was repeated on 7th and 14th day. A fluoride ion selective electrode connected to ion selective electrode meter (Orion 940, Expotech, Texas, USA) was used for measuring fluoride release on day 1, 7, 14 and 21. The calibrations were done according to manufacturer’s instruction using standard fluoride solution at concentrations varying from 0.20, 1.00, 2.00, 10.00, 20.00 and 100 ppm fluoride. One ml of deionized water was taken from each container and analysed for fluoride release after 1:1 dilution with 0.1ml Total Ionic Strength Adjustment Buffer (TISAB) III to provide constant background ion strength, decomplex the fluoride and stabilize the pH of the solution; and fluoride value from each sample was recorded in ppm.
Evaluation of Antibacterial Activity
Twenty samples of each experimental group were tested for the antibacterial activity against Streptococcus mutans (ATCC 25175). For the evaluation of the antibacterial effect agar diffusion test was employed. A base layer containing 15 ml of Muller Hilton agar (Himedia Laboratory Pvt. Ltd., Mumbai, India) for the agar diffusion test was evenly spread to a thickness of 5 mm in sterile petri dish. In each petri dish after the solidification of the culture medium, spreading of bacterial strains was done evenly using the plate spreader. On agar plate, four wells measuring 5.5 mm were made using the blunt end of a micropipette tip. Specimens of experimental groups were prepared according to the manufacturer’s instruction and packed into the well. The wells were completely filled by set specimens. The culture plates were placed in the incubator at 37oC for 48 hours. The diameters of zone of inhibition were recorded by Vernier Calliper in millimeters around the specimens. The greatest distance between the two points were measured at the outer limit of inhibition halo formed around the wells. This measurement was repeated three times and the mean was calculated for each well.
Statistical Analysis
Mean and standard deviation values of fluoride release and zones of inhibition were calculated. Data was further analysed using one-way and two-way Analysis of variance (ANOVA) followed by LSD post-hoc test. The p-value <0.05 was considered statistically significant. Statistical analysis was done using Statistical Package for Social Sciences (SPSS) v.22 for windows.
Results
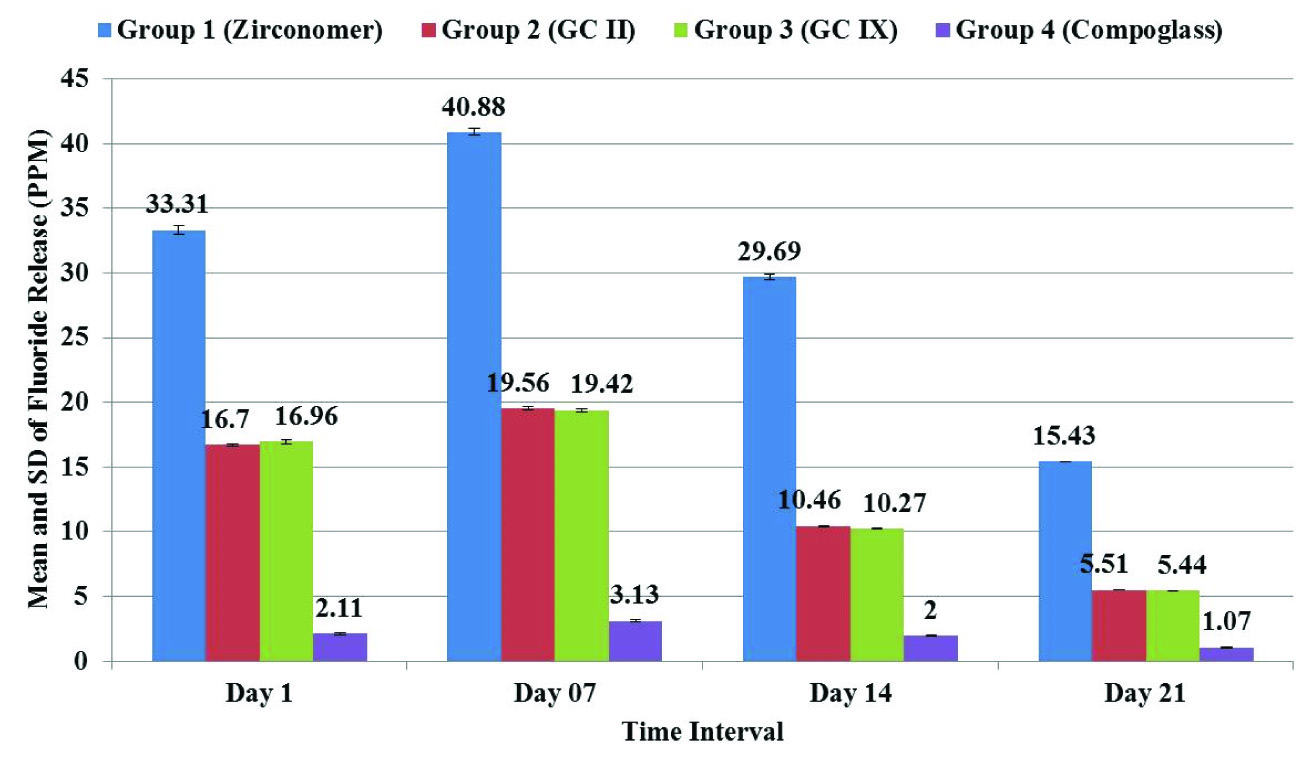
Two-way ANOVA showed significant difference in fluoride release between four cements. When LSD post-hoc test was applied, it showed that at all the time intervals statistically significant maximum fluoride release was observed with group 1 (Zirconomer) and minimum with group 4 (Compoglass). There was no significant difference in fluoride release from group 2 (GC II) and group 3 (GC IX) at all the time intervals [Table/Fig-1,2].
Comparison of Fluoride Release (ppm) at different time intervals from different cements.
| Groups | Fluoride release (mean ± SD, PPM) at different time intervals |
|---|
| Day 1 | Day 07 | Day 14 | Day 21 |
|---|
| Group 1 (Zirconomer) | 33.31 ± 0.32 | 40.88 ± 0.05 | 29.69 ± 0.14 | 15.43 ± 0.08 |
| Group 2 (GC II) | 16.70 ± 0.25 | 19.56 ± 0.09 | 10.46 ± 0.12 | 5.51 ± 0.10 |
| Group 3 (GC IX) | 16.96 ± 0.21 | 19.42 ± 0.04 | 10.27 ± 0.03 | 5.44 ± 0.04 |
| Group 4 (Compoglass) | 2.11 ± 0.01 | 3.13 ± 0.01 | 2.00 ± 0.02 | 1.07 ± 0.04 |
| Two-way ANOVA | F=4748.281, p= <0.001, Significant Difference |
| LSD Post-hoc Test(Sig. Results) | Zirconomer> GC II = GC IX > Compoglass | Zirconomer> GCII = GCIX > Compoglass | Zirconomer > GCII = GCIX > Compoglass | Zirconomer > GCII = GCIX > Compoglass |
Mean and standard deviation of fluoride release (PPM) at different time intervals from different cements.
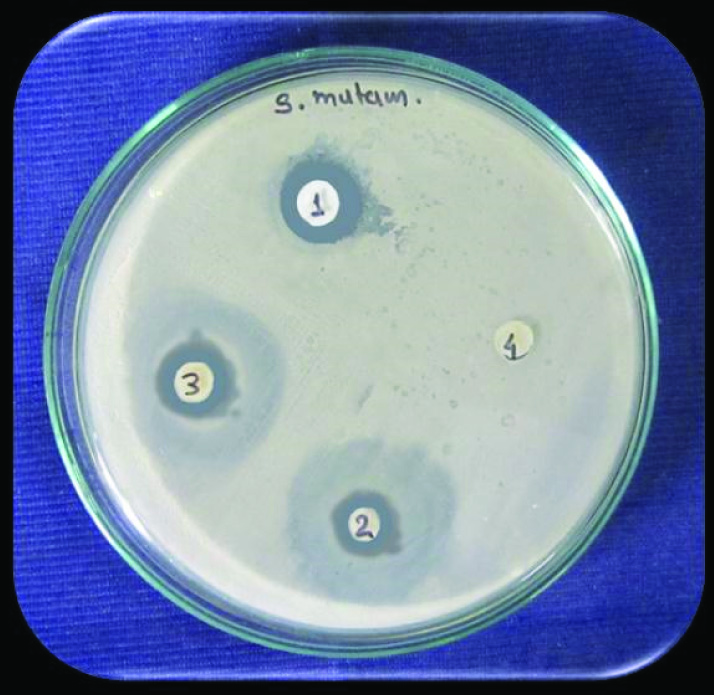
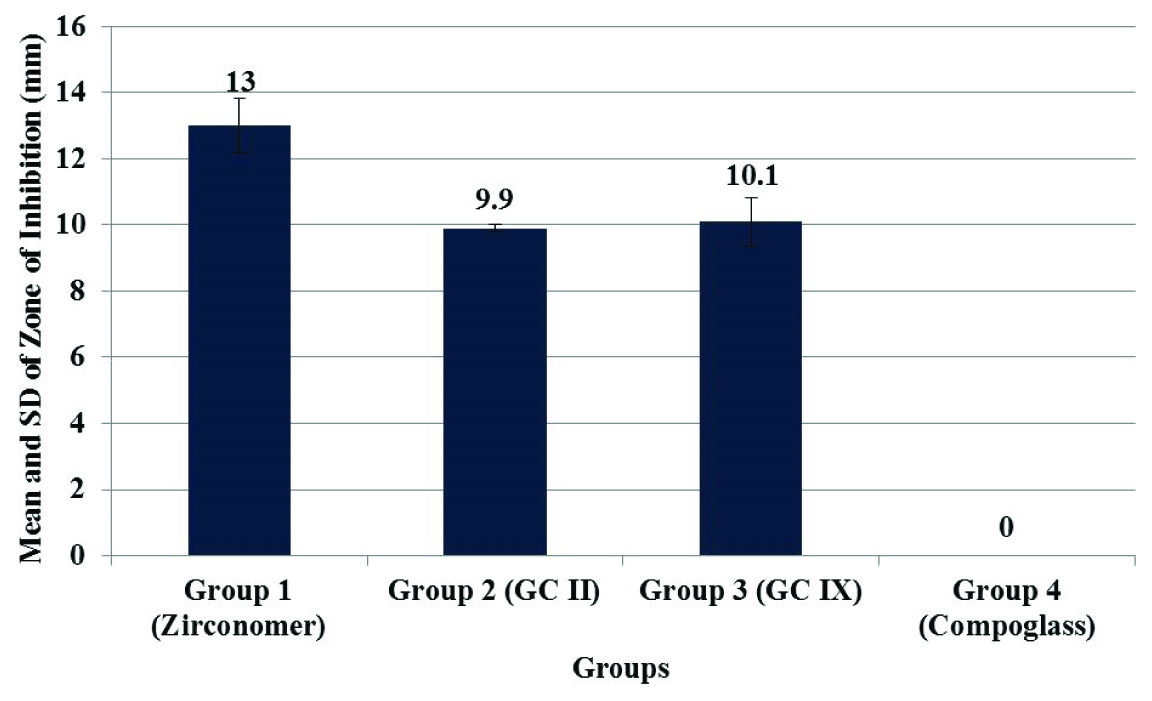
For antibacterial activity, largest zone of inhibition against Streptococcus mutans at 48 hours was observed with group 1 (Zirconomer). There was no zone of inhibition observed with group 4 (Compoglass) [Table/Fig-3]. One-way ANOVA followed by LSD post-hoc test showed that statistically significant largest zone of inhibition was observed with group 1 (Zirconomer) [Table/Fig-4,5].
Zone of inhibition against Streptococcus mutans at 48 hours in different cements.
(1 = Group 1: Zirconia Reinforced Glass Ionomer Cement; 2 = Group 2: Glass Ionomer Cements II; 3 = Group 3: Glass Ionomer Cements IX; 4 = Group 4: Compomer).
Comparison of zone of inhibition against Streptococcus mutans at 48 hours between different cements.
| Groups | Zones of Inhibition (mm)Mean ± SD |
|---|
| Group 1 (Zirconomer) | 13.00 ± 0.83 |
| Group 2 (GC II) | 9.90 ± 0.10 |
| Group 3 (GC IX) | 10.10 ± 0.73 |
| Group 4 (Compoglass) | 0.00 ± 0.00 |
| One-way ANOVA | F=520.269, p= <0.001, Significant Difference |
| LSD Post-hoc Test(Sig. Results) | Zirconomer > GC II = GC IX > Compoglass |
Mean and standard deviation of zone of inhibition against Streptococcus mutans at 48 hours in different cements.
Discussion
To achieve the properties of ideal, there are innovations in the restorative materials [15]. Development of zirconia reinforced GIC (Zirconomer) is one of them. Zirconomer contains zirconia or zirconium oxide which has been used as indirect restorative material (crowns and bridges) since 1998 [13].
In the present study fluoride release and antibacterial activity of GC II (conventional type II glass ionomer cement), GC IX (type IX glass ionomer cement), Compoglass (compomer) and Zirconomer (zirconia reinforced glass ionomer cement) were evaluated against Streptococcus mutans. In literature search we could not find any study on fluoride release and antibacterial properties of Zirconomer. The present research is the first of its kind on Zirconomer.
The complex process of fluoride release from GIC depends on several factors. Intrinsic factors such as formulation, solubility or porosity of the material affects the amount of fluoride release [16]. In restorative materials fluoride content and release should be as maximum as possible without negating effects on physical and mechanical properties and causing unnecessary degradation of the restoration [17]. In the present study at all the time intervals statistically significant maximum fluoride release was observed from Zirconomer and minimum from Compoglass.
Higher amount of fluoride release from Zirconomer [Table/Fig-1,2] may be attributed to its chemical composition, physical properties and consistency of mix (8:1 P/L). Lower amount of fluoride release from compomer was similar to earlier studies [6–8,18]. Fluoride release difference between glass ionomers and compoglass (Compomer) could be due to the fact that after curing and before contact with water, the fluoride in polyacid modified composite is not free, but bound in the filler particles, which are enclosed in the polymerized matrix and in the first phase of setting, polyacid modified composite resin completely behaved like composites [8]. In a 36-months study, authors have found that compomers released relatively lesser fluoride than GICs during the first year, but subsequently, it became equal [7].
In the present study fluoride release from all GICs was first increased till day 7 then decreased on day 14 and 21. These results were similar to many earlier studies [8,15,19]. This initial fluoride “Burst Effect” is a normal feature of GICs which is desired to reduce the viability of bacteria that may have been left in the inner carious dentin and persuade remineralization of enamel/dentin [8,16].
In the second part of present study antibacterial activity of cements was evaluated against Streptococcus mutans at 48 hours [Table/Fig-4,5]. Antibacterial properties of restorative cements have been evaluated in the past, and the bactericidal effects are often attributed to their fluoride release. A variety of mechanisms are involved in the anti-cariogenic effects of fluoride on the teeth. Fluoride inhibits production of bacterial acids and glucans produced by S. mutans, which is known to be the primary aetiologic factor for carious lesions, and therefore has a routine use in testing the antimicrobial activity of restorative materials [20]. In the present study, the antibacterial activity was evaluated using the agar diffusion test. This assay allows bacteria to be screened in a routine, economical, and easy way for the detection of resistance [20].
In the present study largest zone of inhibition was observed with Zirconomer and smallest with compomer (Compoglass). This difference in antimicrobial activity might be related with dissimilarity in release of fluoride from different GICs [19].
Limitations
The present study was conducted in laboratory conditions (invitro study). Oral environment is dynamic and different from invitro conditions. Further invivo studies with more parameters are recommended to evaluate Zirconomer in real environmental circumstances.
Conclusion
Fluoride is a well-known anti-caries agent. Fluoride release from restorative materials is imperative in clinical dentistry. This invitro investigation has revealed that zirconia reinforced glass-ionomer cement (Zirconomer) had maximum antibacterial activity against S.mutans and fluoride release.
[1]. Cho SY, Cheng AC, A review of glass ionomer restorations in the primary dentitionJ Can Dent Assoc 1999 65:491-95. [Google Scholar]
[2]. Faraj BM, Mohammad HM, Mohammad KM, The changes in dentists’ perception and patient’s acceptance on amalgam restoration in Kurdistan-Iraq:a questionnaire-based cross-sectional studyJ Clin Diagn Res 2015 9(4):ZC22-25. [Google Scholar]
[3]. Babar MG, Lin SL, Cariostatic effect of fluoride-containing restorative materials : a reviewMalays Dent J 2009 30(2):130-36. [Google Scholar]
[4]. Meenakshi M, Muralidharan NP, A study to assess the antibacterial property of five endodontic sealersInt J Life Sci Rev 2015 1(12):326-28. [Google Scholar]
[5]. Vermeersch G, Leloup G, Delmee M, Vreven J, Antibacterial activity of glass-ionomer cements, compomers and resin composites: Relationship between acidity and material setting phaseJ Oral Rehabil 2005 32(5):368-74. [Google Scholar]
[6]. Vermeersch G, Leloup G, Vreven J, Fluoride release from glass-ionomer cements, compomers and resin compositesJ Oral Rehabil 2001 28:26-32. [Google Scholar]
[7]. Asmussen E, Peutzfeldt A, Long-term fluoride release from a glass ionomer cement, a compomer and from experimental resin compositesActa Odontol Scand 2002 60:93-97. [Google Scholar]
[8]. Mousavinasab SM, Meyers I, Fluoride release by glass ionomer cements, compomer and giomerDent Res J (Isfahan) 2009 6(2):75-81. [Google Scholar]
[9]. Glass ionomer cements [Internet]. [cited 2016 Jan 17]. Available from: http://www.dentalstrings.com/2011/08/glass-ionomer-cements.html [Google Scholar]
[10]. Kiran A, Hegde V, A short term comparitive analysis of fluoride release from a newly introduced glass ionomer cement in deionised water and lactic acidJ Int Oral Heal 2010 2(2):71-77. [Google Scholar]
[11]. Tyas MJ, Clinical evaluation of glass-ionomer cement restorationsJ Appl oral Sci 2006 14(sp. issue):10-13. [Google Scholar]
[12]. Zirconomer Zirconia Reinforced Restorative[Internet]. [cited 2016 Jan 4]. Available from: http://www.shofu.com.sg/downloads/pdf/Zirconomer Brochure.pdf [Google Scholar]
[13]. Russell G, A closer look at zirconium oxideDent Dialogue 2006 6:108-21. [Google Scholar]
[14]. Mungara J, Philip J, Joseph E, Rajendran S, Elangovan A, Selvaraju G, Comparative evaluation of fluoride release and recharge of pre-reacted glass ionomer composite and nano-ionomeric glass ionomer with daily fluoride exposure: an invitro studyJ Indian Soc Pedod Prev Dent 2013 31(4):234-39. [Google Scholar]
[15]. Prabhakar AR, Prahlad D, Kumar SR, Antibacterial activity, fluoride release and physical properties of an antibiotic modified glass ionomer cementPediatr Dent 2013 35:411-15. [Google Scholar]
[16]. Upadhyay S, Rao A, Shenoy R, Comparison of the amount of fluoride release from nanofilled resin modified glass ionomer, conventional and resin modified glass ionomer cementsJ Dent (Tehran) 2013 10(2):134-40. [Google Scholar]
[17]. Forsten L, Fluoride release and uptake by glass-ionomers and related materials and its clinical effectBiomaterials 1998 19(6):503-08. [Google Scholar]
[18]. Yildiz M, Bayindir YZ, Fluoride release from conventional glass-ionomer cements and polyacid-modified composite resinsFluoride 2004 37(1):38-42. [Google Scholar]
[19]. Shashibhushan KK, Basappa N, Subba Reddy VV, Comparison of antibacterial activity of three fluorides- and zinc-releasing commercial glass ionomer cements on strains of mutans streptococci: an invitro studyJ Indian Soc Pedod Prev Dent 2008 26(Suppl 2):S56-61. [Google Scholar]
[20]. Hotwani K, Thosar N, Baliga S, Bundale S, Sharma K, Antibacterial effects of hybrid tooth colored restorative materials against Streptococcus mutans: an invitro analysisJ Conserv Dent 2013 16(4):319-22. [Google Scholar]