Quantitative Assessment of Tumor Associated Macrophages in Head and Neck Squamous Cell Carcinoma Using CD68 Marker: An Immunohistochemical Study
Neeta Bagul1, Souparna Roy2, Anjali Ganjre3, Rahul Kathariya4, Aishwarya Meher5, Pratibha Singh6
1 Professor and Head, Department of Oral Pathology and Microbiology, Vyas Dental College and Hospital, Jodhpur, Rajasthan, India.
2 Consultant Dental Surgeon, Calcutta Institute of Maxillofacial Surgery and Research, Kolkata, West Bengal, India.
3 Lecturer, Department of Oral Pathology and Microbiology, Dr. D Y Patil Dental College and Hospital, Dr. D Y Patil Vidyapeeth, Pune, Maharashtra, India.
4 Lecturer, Department of Periodontology, Dr. D. Y Patil Dental College and Hospital, Dr. D. Y Patil Vidyapeeth, Pune, Maharashtra, India.
5 Intern, Department of Oral Pathology and Microbiology, School of Dentistry, D. Y. Patil University, Nerul, Navi Mumai, Maharashtra, India.
6 Lecturer, Department of Oral Pathology, D. Y Patil Dental School, D. Y Patil, Knowledge City, Lohegaon, Pune, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rahul Kathariya, Lecturer, Department of Periodontology, Dr. D. Y Patil Dental College and Hospital, Dr. D. Y Patil Vidyapeeth, Pune-411018, Maharashtra, India.
E-mail: rkathariya@gmail.com
Introduction
Oral Squamous Cell Carcinoma (OSCC) is one of the most prevalent cancers in India. Clear evidence regarding inflammation being an etiological factor of cancer was found only in the last few decades. A major inflammatory component in the tumor tissue is Tumor-Associated Macrophages (TAMs). The CD68 antibody is a marker for staining TAMs.
Aim
The aim of this study is to quantify the macrophage count in healthy oral mucosa and OSCC and comparing TAMs in different histopathological grades of OSCC immunohistochemically.
Materials and Methods
Thirty archival specimens of OSCC patients and 10 healthy biopsy samples were collected. Immunohistochemical staining was done using a CD68 marker. Statistical analysis was done using Kruskal-Wallis ANOVA and Mann-Whitney U test.
Results
Comparing CD68 expression in various study groups showed a significant difference (p=0.000). The pair-wise analysis showed different grades of OSCC, which differed significantly for CD68 expression from the normal oral mucosa.
Conclusion
The most significant cells present in tumor stroma are TAMs, which remain in close proximity to neoplastic cells and interact with them via several chemical mediators, which may serve to increase the invasiveness of the malignant epithelium. Dense infiltration of TAMs adjacent to tumor cells and islands vividly implies their role in tumor progression.
CD68 antigen, Oral squamous cell carcinoma, Reactive oxygen species, Tumorigenesis
Introduction
Oral Cancer (OC) occurring in India accounts for 57.5% of all global occurrences [1]. The European Union registers about 40,000 new cases per year while 30,000 new cases are registered annually in the United States [2]. In South-Asia OSCC is found to be the most common cause of cancer-related deaths [3]. This high prevalence is mainly because of region-specific epidemiological factors, like tobacco and betel quid chewing,
The first possible link between cancer and an inflammatory tissue microenvironment was noticed by Rudolf Virchow in the 19th century, but clear evidence regarding the role of inflammation was found only in the last few decades [4]. It has been observed that along with promoting tumor development, tobacco, also produces chronic inflammation which facilitates tumorigenesis [5].
One of the major inflammatory components in the tumor tissue is TAMs. Macrophages can be grouped into two types, one that is normally present in inflamed tissue (M1 phenotype) and the other that is present in cancer-related inflammation (M2 phenotype). The classical M1 phenotype macrophages are part of the immune system, intricately involved in processes such as phagocytosis and production of inducible Nitric Oxide Synthase (iNOS) and Reactive Oxygen Species (ROS) serving to protect the organism from harmful pathogens. On the other hand, macrophages that are of the M2 prototype are produced by chemokines and polarizing cytokines, released by tumor cells and thus are able to evade the immune system ensuring their escape from destruction and subsequently they proliferate [6]. Thus, the aim of the study was to evaluate and quantify CD68 antibody (a marker for staining TAMs) in normal tissue and OSCC using immunohistochemistry.
Materials and Methods
Thirty archival (excisional biopsy) specimens of formalin-fixed-paraffin-embedded tissue blocks of OSCC patients were retrieved from the Department of Oral Pathology & Microbiology, Dr. D. Y. Patil Dental College & Hospital, Pune, for the study. Sections were stained by H & E to differentiate between different grades of OSCC [Table/Fig-1,2 and 3]. Ten biopsy samples for the control group were obtained from patients undergoing esthetic gingivoplasty, (after thorough oral prophylaxis and reduction of gingival inflammation). The study was approved by the Scientific and Ethical Committee of the Institution. Written informed consent was obtained from the patients prior to taking his/her tissue for this study.
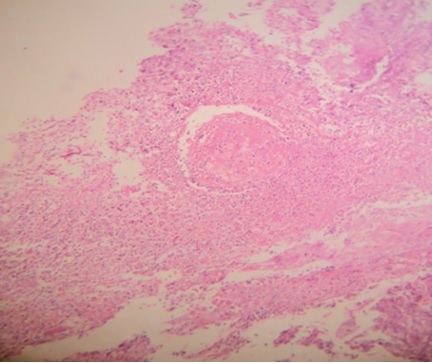
H & E section of well differentiated squamous cell carcinoma with keratin pearl (10x).
H & E section of moderately differentiated squamous cell carcinoma (10x).
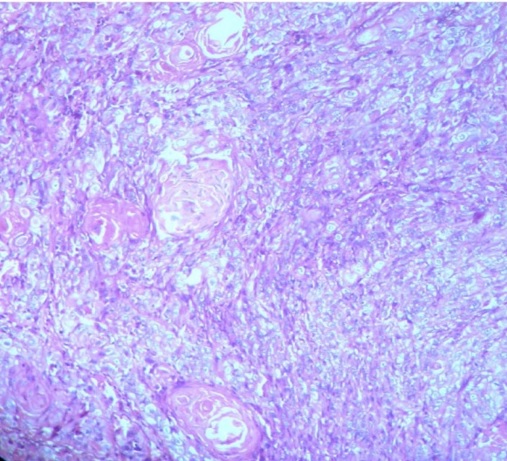
H&E stained section of a poorly differentiated oral squamous cell carcinomatous tissue (40x).
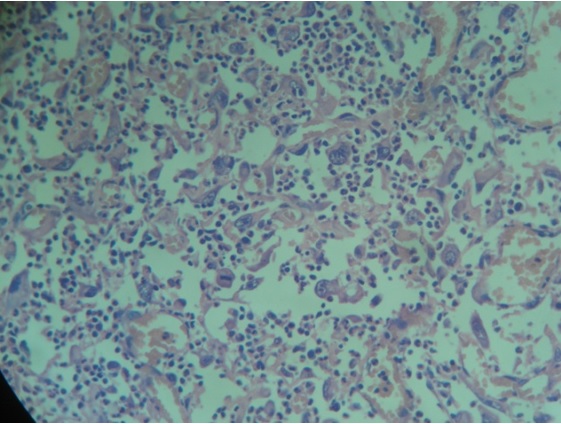
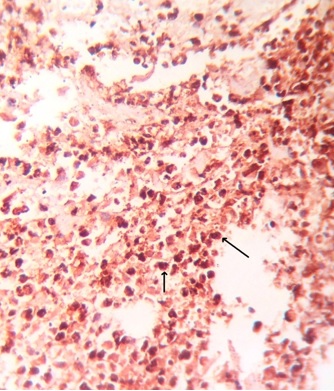
Immunohistochemical Staining: A 5μm-thick paraffin section was taken on lysine-coated slides and was stained immunohistochemically using mouse monoclonal antibodies to CD68 (Thermo Fisher Scientific, MS-397; Lab Vision Corporation, Fremont, CA, USA). Primary antibody was used in 1:200 dilutions (as per product instructions for use). Before treatment with the primary antibody, tissue sections were subjected to enzyme digestion for 5 minutes at 37°C with Protease XXV at 1mg/ml PBS [Lab Vision Catalog # AP 9004]. The CD68 stained slides so obtained were observed under a light microscope at low magnification (10X) [Table/Fig-4] for three ‘Hot Spots’ i.e. areas where the density of CD68 positive cells was recorded maximum by two observers NB and SR independently. The examiners were considered calibrated once a statistically significant correlation and statistically non-significant difference between duplicate measurements were obtained (r=0.92). These ‘Hot Spots’ were then seen under high magnification (40X) [Table/Fig-5] using a light microscope and CD68 positive cells were counted and the mean value was obtained. Values thus obtained were graded as follows: Of the hundred cells counted, GRADE 0: Single few cells positive; GRADE+1: Less than 10% of the total number of cells in that hot spot were positive; GRADE+2: More than 10% & less than 50% cells positive; GRADE+3: More than 50% cells positive.
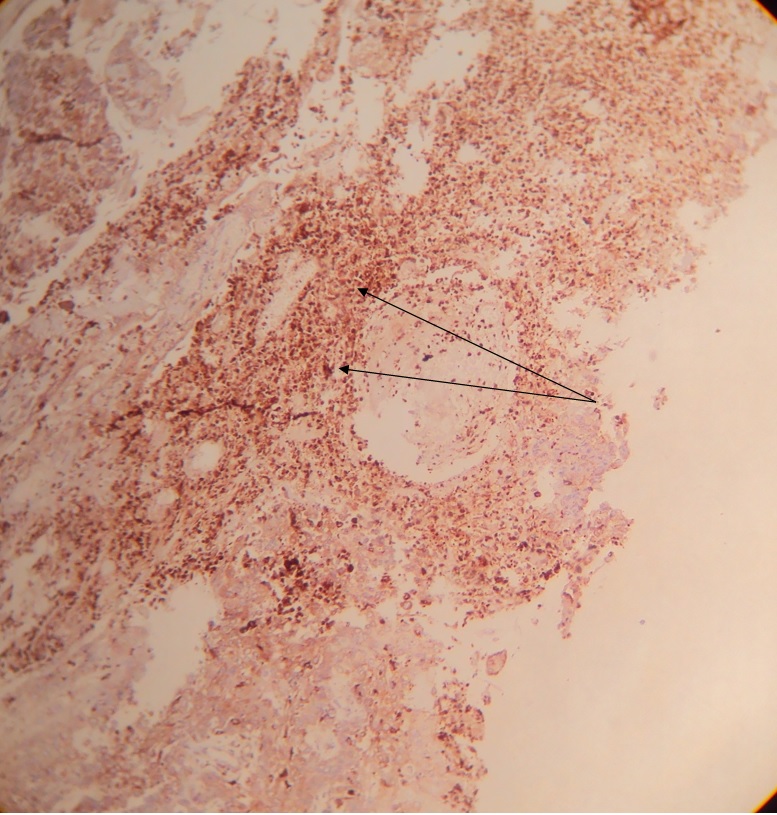
A 10x magnification of a CD68 stained section of a well differentiated OSCC showing hot-spots (dense infiltration) of CD68 +ve tumor-associated macrophages (shown with arrows).
A 40x magnification of a well differentiated OSCC showing CD68 positive tumor-associated macrophages (shown with arrows).
Statistical Analysis
All values were subjected to statistical analysis using Kruskal-Wallis ANOVA and Mann-Whitney U test. All statistical analyses were completed using SPSS version 20.0 software. A p value of <0.05 indicated statistical significance.
Results
The comparison of CD68 expressions in the various differentiations of OSCC groups is tabulated using Kruskal-Wallis ANOVA [Table/Fig-6]. The Chi-Square value of 28.824 and p-value of 0.000 was observed which is statistically highly significant. The pair-wise analysis using Mann-Whitney U test [Table/Fig-7] showed that all the grades of OSCC differed significantly with respect to the expression of CD68 from the normal oral mucosa (control group), whereas, there was no statistical difference in the expression of the biomarker between the various grades of OSCC.
Comparison of CD68 expression in the study groups using Kruskal Wallis ANOVA.
| Group | N | Mean rank | Chi-square value | p-value |
|---|
| Well differentiated OSCC | 10 | 27.00 | 28.824 | 0.000** |
| Moderately diffentiated OSCC | 10 | 24.00 |
| Poorly differetiated OSCC | 10 | 25.50 |
| Normal mucosa | 10 | 5.50 |
** Highly significant
Pair wise comparison of the study groups using Mann Whitney U test.
| Group | Mean rank | Mann Whitney U | p-value |
|---|
| Well differentiated OSCC | Moderately differentiated OSCC | 40.00 | 0.276 |
| Poorly differentiated OSCC | 45.000 | 0.542 |
| Normal mucosa | 0.000** | 0.000** |
| Moderately differentiated OSCC | Poorly differentiated OSCC | 45.000 | 0.615 |
| Normal mucosa | 0.000** | 0.000** |
| Poorly differentiated OSCC | Normal mucosa | 0.000** | 0.000** |
** Highly significant
Discussion
A wide range of cells are found in the microenvironment of OSCC tissue, consisting of both epithelial (malignant) and stromal cells. It has been observed that the dynamic interplay between these cells is responsible for tumor progression. TAMs constitute a dominant portion of the leukocyte population in tumor stroma [7].
Areas of hypoxia will occur within a solid tumor which has grown more than 2mm due to insufficiencies in the simple diffusion of oxygen as well as nutrients to metabolizing tissues. In such areas, several factors such as Monocyte Chemotactic Protein 1 (MCP-1) / CCL2 and Granulocyte-Macrophage-Colony-Stimulating Factor (GMCSF) are produced which in turn help to recruit monocytes continually within the tumor microenvironment. These accumulate in hypoxic areas after they differentiate into TAMs [7,8].
The presence of TAMs was observed in all the OSCC specimens in our study and an increased TAM infiltration around the neoplastic and malignant epithelial islands and cells was seen. This may certainly denote the recruitment of TAMs within the tumor microenvironment and their potential role in modifying the neoplastic biological behavior of the tumor. This may also suggest that the TAMs are involved in tumor cytotoxicity as well as scavenging of tumor cell debris.
Lu CF et al., in their study with 92 OSCC tissue specimens found the infiltrating macrophage count to be significantly higher in OSCC tumors with a large tumor size, positive lymph node metastasis and more advanced clinical stages or recurrence [8, 9]. Our findings are in accordance with them as we also found significant macrophage count in OSCC specimens and the tissues of patients with lymph node metastasis. Dai T et al., in their sample of 42 cases of OSCC and 10 normal tissues found that there were masses of macrophage infiltration in OSCC [9,10], which is in agreement with their previous findings as well as the findings in our study. El-Rouby DH in a study observed that the CD68 positive TAMs were distributed within the connective tissue surrounding the cancer cells [10,11] and similar observations were found in our study.
However, our findings are different from the study by Lo Muzio L et al., [11,12] with respect to tumor differentiation. We found no statistically significant difference in the expression of CD68 biomarker between the various histopathological grades of OSCC on using Mann-Whitney U test while their results showed a trend for the association of inflammatory infiltrates with the degree of tumor differentiation: well and moderately differentiated tumors showed associations of dense inflammatory infiltrate while poorly differentiated cancers seem to be associated to a low inflammatory infiltrate.
Since it is usually observed that TAMs are located in the stroma surrounding cancer cells, this localization of TAMs promotes angiogenesis, as a number of molecules with possible impact on angiogenesis have been shown to be expressed by macrophages in low oxygen conditions, such as Vascular Endothelial Growth Factor (VEGF), Tumor Necrosis Factor (TNF)-α and Basic Fibroblast Growth Factor (bFGF) [7].
Mantovani A et al., have proposed that induction of TAMs and their polarization into the M2 phenotype may be caused due to the exposure of macrophages to IL4 and IL10 in tumor microenvironments [12, 13]. It has been shown that the invasive properties of tumor cells get enhanced when tumor cells are co-cultured with macrophages in a manner that is dependent on TNF-α and matrix metalloproteinases as reported by Hagemann T et al., [13, 14]. The proliferation of tumor cells and infiltration by TAMs have positive correlations in various carcinomas as shown in studies measuring Ki67 indices in endometrial carcinomas [14, 15], the mitotic index in renal cell carcinomas [15, 16] and levels of MIB-1 in breast carcinomas [16, 17]. It seems that TAMs play dual roles in the process of metastasis by aiding the release of the primary tumor cells that will metastasize and also in establishing the secondary tumor at a distant site [17, 18]. It has been shown that infiltration of TAMs was regulated by hypoxia inducing factor semaphoring [19]. Also, TAMs are responsible for cancer progression, they initiate cancer progression via ‘angiogenic switch’ by releasing proangiogenic cytokine VEGF-A. Formation of new blood vessels within the tumor microenvironment provides nutrition and oxygen to fast growing tumor cells, at the same time they help in the escape of tumor cells through the newly formed blood vessels which initiate metastasis [19]. Recent studies have indicated a clear association of roles played by TAMs and Cancer Stem Cells (CSC). Markers of TAMs like CD163 have been found to be overexpressed along with CD68 in OSCC tissue compared to normal tissue and this suggests that CD68 is an important diagnostic and prognostic marker for OSCC [20]. An association between TAM and CSC markers has been established. The aggressiveness in biological behavior of the tumor can be implied by the expression of these markers [20]. There have been studies indicating that micro-localization of TAMs can serve as an important prognostic indicator in OSCC [21]. It has been observed in murine studies that the systemic depletion of macrophages in Polyomavirus Middle T Antigen (PyMT) induced mammary cancer, results in a decrease in lung metastasis indicating the role of TAMs in the process [22]. A meta-analysis study by Zhang QW et al., has found that TAMs seem to have an association with poor survival in gastric, urogenital, and head and neck cancer patients [23].
Recent work on TAMs indicates that strictly classifying them into two phenotypes M1 and M2 as mentioned before is an over simplification since they are not static [24] and phenotypical TAMs show plasticity and can display a number of activation states ranging between M1 and M2 [6]. However, infiltration of M1 macrophages is accompanied by an increase in the infiltration of macrophages with M2 phenotype. Hence, according to stage-dependent manner/grading they can be correlated with better prognosis [25]. So any one biomarker is insufficient to identify the dynamicity shown by TAMs in a variable and ever-changing tumor microenvironment. Thus, a cocktail of biomarkers along with modern sophisticated and advanced immunohistochemistry techniques need to be identified which will serve the purpose of identification and quantification of the entire range of phenotypically different TAMs. Moreover, correlating the TAM count with clinical findings may reveal the exact role of TAMs in the prognosis of the disease.
Limitation
Further investigations are needed to elucidate the mechanism for regulation of extracellular matrix by TAMs and other stromal cells in human malignancies, and this may help for the design of novel therapeutic strategies in future.
Conclusion
TAMs form a significant portion of tumor stroma and are mainly presented adjacent to the tumor cells. It has become clear now that within the tumor microenvironment, not only the neoplastic epithelial cells but also the stromal cells have a major role to play in the biological behavior and progression of cancer. The significance of TAMs which remain in close proximity to the tumor cells or interacting with the tumor cells with the help of several chemical mediators cannot be overlooked. The dense infiltration of TAMs adjacent to the islands of cancer cells vividly implies its role in tumor progression. Thus, only by improving our understanding of cells within the tumor stroma and its cellular elements can we hope to plan better and effective therapeutic strategies for the treatment of OSCC.
** Highly significant
** Highly significant
[1]. Chaturvedi P, Head and neck surgeryJ Can Res Ther 2009 5:143 [Google Scholar]
[2]. Hunter KD, Parkinson EK, Harrison PR, Profiling early head and neck cancerNat Rev Cancer 2005 5:127-35. [Google Scholar]
[3]. Paterson IC, Eveson JW, Prime SS, Molecular changes in oral cancer may reflect etiology and ethnic originEur J Cancer B Oral Oncol 1996 32B:150-53. [Google Scholar]
[4]. Grivennikov SI, Greten FR, Karin M, Immunity, inflammation, and cancerCell 2010 140:883-99. [Google Scholar]
[5]. Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M, Tobacco smoke promotes lung tumorigenesis by triggering IKK beta and JNK1-dependent inflammationCancer Cell 2010 17:89-97. [Google Scholar]
[6]. Heusinkveld M, van der Burg SH, Identification and manipulation of tumor-associated macrophages in human cancersJ Transl Med 2011 9:216-29. [Google Scholar]
[7]. Lee CC, Liu KJ, Huang TS, Tumor-associated macrophage: its role in tumor angiogenesisJ. Cancer Mol 2006 2:135-40. [Google Scholar]
[8]. Deshmane SL, Kremlev S, Amini S, Sawaya BE, Monocyte chemoattractant protein-1 (MCP-1): an overviewJ Interferon Cytokine Res 2009 29(6):313-26. [Google Scholar]
[9]. Lu CF, Huang CS, Tjiu JW, Chiang CP, Infiltrating macrophage count. A significant predictor for the progression and prognosis of oral squamous cell carcinomas in TaiwanHead and Neck 2010 32:18-25. [Google Scholar]
[10]. Dai T, Song Y, Ma H, Feng H, Studies on the expression of MMP-9 and significance of a macrophage assay in oral squamous cell carcinomaChinese J Clin Oncol 2007 4:333-37. [Google Scholar]
[11]. El-Rouby DH, Association of macrophages with angiogenesis in oral verrucous squamous cell carcinomasJ Oral Pathol Med 2010 39:559-64. [Google Scholar]
[12]. Lo Muzio L, Santoro A, Pieramici T, Bufo P, Di Alberti L, Mazzotta P, Immunohistochemical expression of CD3, CD20, CD45, CD68 and bcl-2 in oral squamous cell carcinomaAnal Quant Cytol Histol 2010 32:70-77. [Google Scholar]
[13]. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A, Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytesTrends Immunol 2002 23:549-55. [Google Scholar]
[14]. Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, Binder C, Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-a dependent up-regulation of matrix metalloproteasesCarcinogenesis 2004 25:1543-49. [Google Scholar]
[15]. Wang FQ, So J, Reierstad S, Fishman DA, Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinaseInt J Cancer 2005 114:19-31. [Google Scholar]
[16]. Hamada I, Kato M, Yamasaki T, Iwabuchi K, Watanabe T, Yamada T, Clinical effects of tumor-associated macrophages and dendritic cells on renal cell carcinomaAnticancer Res 2002 22:4281-84. [Google Scholar]
[17]. Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S, Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel densityOncol Rep 2005 14:425-31. [Google Scholar]
[18]. Lewis CE, Pollard JW, Distinct role of macrophages in different tumor microenvironmentsCancer Res 2006 66:605-12. [Google Scholar]
[19]. Riabov V, Gudima A, Nan Wan, Mickley A, Orekhov A, Kzhyshkowska J, Role of tumor associated macrophages in tumorangiogenesis and lymphangiogenesiswww.frontiersin.org 2015 5(75):1-13. [Google Scholar]
[20]. Wehrhan F, Büttner-Herold M, Hyckel P, Moebius P, Preidl R, Distel L, Increased malignancy of oral squamous cell carcinomas (OSCC) is associated with macrophage polarization in regional lymph nodes- an immunohistochemical studyBMC Cancer 2014 14:522 [Google Scholar]
[21]. Ni YH, Ding L, Huang XF, Dong YC, Hu QG, Hou YY, Microlocalization of CD68+ tumor-associated macrophages in tumor stroma correlated with poor clinical outcomes in oral squamous cell carcinoma patientsTumor Bio 2015 (doi 10.1007/s13277-015-3189-5) [Google Scholar]
[22]. Lin EY, Nguyen AV, Russell RG, Pollard JW, Colony-stimulating factor 1 promotes progression of mammary tumors to malignancyJ Exp Med 2001 193:727-40. [Google Scholar]
[23]. Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literaturePLoS One 2012 7:e50946 [Google Scholar]
[24]. Bavle RM, From the Editor’s deskJ Oral Maxillofac Pathol 2015 19:3 [Google Scholar]
[25]. Sica A, Mantovani A, Macrophage plasticity, and polarization: in vivo veritasJ Clin Invest 2012 122(3):787-95. [Google Scholar]