In the past, children had received inadequate analgesia due to lack of basic understanding or fear of addiction [1]. After understanding the pain pathways and assessment of pain, quality of analgesia in infants and children has gained special importance [2].
Caudal analgesia was first described and demonstrated by Meredith Campbell for paediatric cystoscopies way back in 1933 since then many advancements have been done in caudal anaesthesia with different concentrations of local anaesthetic agents and adjuvants also been studied for prolonging the analgesic effect [3]. Ropivacaine has been used for caudal analgesia in children and duration is similar to that of Bupivacaine (in equivalent doses) but the onset of motor block is slower, less intense and shorter in duration for a given level of sensory block with less cardiac and central nervous system toxicity [4]. To get adequate analgesic level we need more volume of ropivacaine which can increase the possibility of side effects so many adjuvants have been tried [5–7] to decrease the local anaesthetic volume and concentration. Clonidine alpha2-adrenergic agonist produces analgesia has been studied as an adjuvant to local anaesthetics so that it decreases concentration of local anaesthetic while providing better analgesia without significant side effects [8]. Different doses of clonidine and local anaesthetic agents in a mixture have been tried to get good analgesia [9,10]. So our study was conducted to compare the duration of postoperative analgesia, haemodynamic safety and adverse effects of caudal Ropivacaine and caudal Ropivacaine with Clonidine in different concentrations than in previous studies for better analgesia and minimal side effects in paediatric patients.
Materials and Methods
After Institutional ethical committee approval and a written informed consent from the parents this prospective, double-blind, randomized, controlled study was conducted in Goa Medical College Hospital, Goa from 2012 to 2013. Sixty patients of ASA 1 children between 2-10 years of age, of either sex, scheduled to undergo infraumbilical surgeries (circumcision, herniotomy and orchidopexy) were selected in order to compare the duration of postoperative analgesia, haemodynamic stability and side effects of the study drugs. The patients were randomly allocated into two groups of 30 each: Group A, Group B by sealed envelope technique.
Group A: Received mixture of 0.2% Ropivacaine and normal saline.
Group B: Received mixture of 0.2% Ropivacaine and preservative free Clonidine 1μg/kg.
The total volume of solution was made up to 1ml/kg in both the groups. The attending anaesthesiologist administered the appropriate drug according to the code in the envelope. Name, age, hospital number, diagnosis and procedure were written in the same envelope, sealed and handed over to the investigator at the end of the procedure. A second observer did the patient assessment and data collection. The anaesthesiologist who was collecting the data was blinded to the contents of the study drug. After all the cases had been completed at the end of the study, the code was broken, study drug contents revealed, and data compiled. Patients with history /findings suggestive of Local infection, Bleeding diathesis, Pre-existing neurologic or obvious spinal diseases were excluded from the study.
All patients received 0.5mg/kg of midazolam orally as premedication 30 minutes prior to induction. Patients were connected with ECG, non invasive blood pressure and pulse oximeter for intraoperative monitoring. Baseline haemodynamic parameters e.g., Heart Rate (HR), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP) Oxygen Saturation (SPO2) were obtained. Patients were induced with increasing concentration of sevoflurane (0-8%) in 60% nitrous oxide and 40% oxygen mixture using face mask connected to Jackson Rees circuit. Intravenous access was secured after induction. An infusion of Ringer Lactate was administered according to the requirement.
The airway was maintained either using an appropriate sized facemask or laryngeal mask airway according to the discretion of the attending anaesthesiologist. Anaesthesia was maintained with 2-3% sevoflurane in oxygen-nitrous oxide (40:60) mixture.
After induction of anaesthesia, the patients were placed in left lateral position and a single shot caudal block was performed with full aseptic precautions using a 23G hypodermic needle. Aspiration was done to exclude dural puncture or vessel puncture and the drug was then injected. The site of injection was covered with a sterile dressing and the child was placed supine. Surgery was started 5 minutes after caudal injection.
Anaesthesia was subsequently maintained with oxygen 40%, nitrous oxide 60% and sevoflurane (2-3%). No intravenous analgesics or sedatives were administered.
After caudal anaesthesia haemodynamic changes were monitored every 5 minutes interval for 30 minutes and then 15 minutes interval till the end of operation. Postoperatively same parameters were monitored and recorded every 15 minutes for 2 hours in the post-anaesthetic care unit.
Intraoperatively analgesic efficacy was defined by haemodynamic stability. This was indicated by absence of an increase in heart rate or MAP to > 15% of pre incision baseline values and increase in heart rate or mean arterial pressure >15% and analgesic failure was indicated by the presence of above mentioned haemodynamic parameters within 15 minutes of start of surgery and cases were excluded from the study after giving analgesia with 2 μg/kg of fentanyl. Anaesthetic agents were discontinued at the beginning of skin closure. The duration of surgery was noted in both the groups. At the end of surgery no prophylactic pain relief was given and patients were transferred to post-anaesthetic care unit. Monitoring was continued for vital parameters for 24 hours. In addition pain score, sedation score and motor blockade were assessed. The heart rate, mean arterial pressure, oxygen saturation, pain score and sedation score were assessed for 2 hours in the post-anaesthetic care unit. The same parameters were monitored at 2,4,6,8,10,12,18 and 24 hour in the ward.
Motor block was assessed at the end of surgery, two hours in the post-anaesthetic care unit and at the end of six hours to detect any residual motor weakness by means of motor block score by using Bromage scale [Table/Fig-1]. Failure of oral commands were followed by tactile stimulus to lower limb to get a response [11].
| Grade | Criteria | Degree of block |
|---|
| I | Free movement of legs and feet | Nil (0%) |
| II | Just able to flex knees with free movement of feet | Partial (33%) |
| III | Unable to flex knees, but with free movement of feet | Almost complete (66%) |
| IV | Unable to move legs or feet | Complete (100%) |
Assessment of pain was done by FLACC [Table/Fig-2] score at 2,4,6,8,10,12,18 and 24 hours postoperatively. Duration of adequate analgesia (defined as the time from caudal injection to the first time the FLACC pain score was noted to be 4 or more) was also recorded. Rescue medication was administered in the form of oral syrup of paracetamol 15mg/kg when the FLACC score was > 4. The number of doses of rescue medication required and the time to first administration.
Pain scoring FLACC scale.
| Parameter | 0 | 1 | 2 |
|---|
| Face | No expression | Occasional grimace | Frequent to constant quivering chin. |
| Legs | Normal position or relaxed | Uneasy, restless, tense | Kicking or legs drawn up |
| Activity | Lying quiet | Squirming, shifting back and forth, tense | Arched, rigid or jerking |
| Cry | No cry | Moans or whimpers | Crying steadily |
| Consolability | Content, relaxed | Reassurance, hugging | Difficulty to console |
Sedation was assessed by sedation score 0- awake, 1- arousable by voice, 2- arousable to pain, 3- unarousable. Score: 0 = no pain; 1-3 mild pain; 4-7 moderate pain; 8-10 severe pain
All the patients were observed for adverse effects in the postoperative period for 24 hours including nausea, vomiting, respiratory depression, residual motor blockade, urinary retention, hypotension and bradycardia. A decrease in mean arterial pressure or decrease in heart rate >30% of baseline was called as Hypotension or Bradycardia respectively and was treated by IV fluids, ephedrine or atropine accordingly. Respiratory depression was defined as a decrease in SpO2 to <93% requiring supplemental oxygen via face mask.
Statistical Analysis
The data thus obtained was statistically analysed using software SPSS (V 16) data were analysed using descriptive statistics (means, standard deviation, percentages) Unpaired t-test, Mann-whitney test. The p-value of < 0.05 was considered to be statistically significant.
Results
Pain score was assessed by FLACC scale [Table/Fig-2] at 2,4,6,8,10,12,18 and 24 hours postoperatively. Duration of adequate analgesia (defined as the time from caudal injection to the first time the FLACC pain score was noted to be 4 or more) was also recorded. Rescue medication was administered when the FLACC score was 4 or more. Demographic data [Table/Fig-3]. The groups were comparable with respect to age, weight, sex and duration of surgery difference was found to be insignificant. Pain scores > 4 and timings [Table/Fig-4]
Demographic characterstics.
| Characteristic | Group A(30)Mean+SD | Group B(30)Mean+SD | p-value |
|---|
| Mean age (y) | 4.7 + 1.7 | 4.8 + 1.7 | 0.8206 |
| Mean weight (kg) | 13.43 + 3.14 | 13.73 + 2.8 | 0.6975 |
| Mean duration of Surgery (minutes) | 40 + 8.2 | 40.83 + 7.9 | 0.6912 |
| Gender M/F | 26/4 | 25/5 | >0.05 |
Data are mentioned as mean ±standard deviation M-Male F – Female
Pain score ≥ 4 at various time intervals.
| Time interval (hours) | Group A | Group B | p-value |
|---|
| 2 | 0 | 0 | - |
| 4 | 2(6.6%) | 0 | >0.05 |
| 6 | 18(60%) | 0 | <0.01 |
| 8 | 10(33.3%) | 2(6.6%) | <0.01 |
| 10 | 5(16.6%) | 6(20%) | >0.05 |
| 12 | 16(53.3%) | 16(53.3%) | >0.05 |
| 18 | 11(36.6%) | 9(30%) | >0.05 |
| 24 | 2(6.6%) | 3(10%) | >0.05 |
Data represented absolute numbers (percentages) and p-values at each intervals
None of the children in both the groups had a pain score of ≥ 4 at the end of 2 hours. At the end of 4th hour, 6.6% of children in Group A and none of the children in Group B had a pain score of ≥ 4. At the end of 6th hour, 60% of children in Group A and none of the children in Group B had a pain score of ≥ 4 (p<0.01). At the end of 8th hour, 33.33% of children in Group A and 6.6% of the children in Group B had a pain score of ≥ 4. The difference was statistically significant between the 2 groups at 6th and 8th hour.
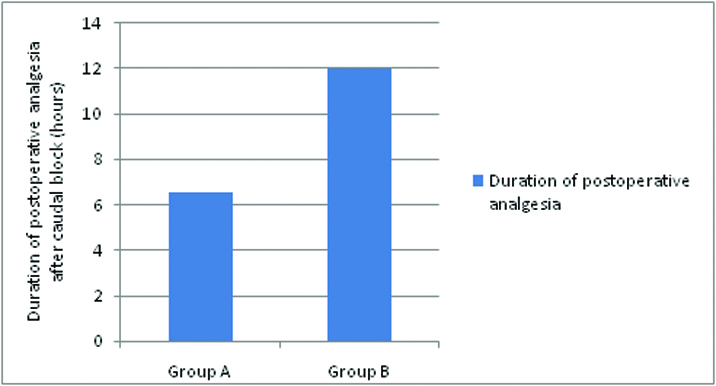
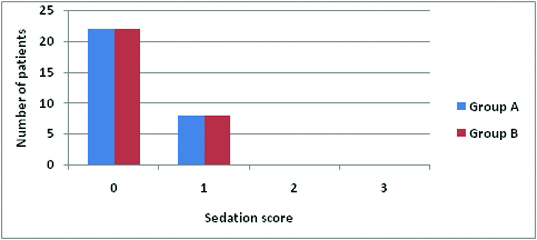
The mean duration of analgesia [Table/Fig-5,6] in Group A was 6.53 ± 1.16 hours and in Group B was 12 ± 2.22 hours. The duration of postoperative analgesia in group B was longer than in group A. This was statistically highly significant with p-value of < 0.001. No motor blockade was observed in both the groups in postoperative period [Table/Fig-5]. In Group A, 4 patients received 1 dose of rescue analgesia, 18 patients received 2 doses of rescue analgesia and 8 patients received 3 doses of rescue analgesia and Group B patients required minimal rescue analgesia, 24 patients received only one rescue analgesia and 6 received 2 doses which is highly significant (p <0.01), All the patients were observed for adverse effects in the postoperative period for 24 hours the incidence of nausea and vomiting was present in 4 patients (13.3%) in Group A compared to 3 patients (10%) in Group B [Table/Fig-5]. However, this was statistically insignificant (p >0.05). Sedation scoring [Table/Fig-7]: 22 patients of each group had a postoperative sedation score of 0 in the first 2 hours. 8 patients of each group had a postoperative sedation score of 1 in the first 2 hours. There was no significant difference (p > 0.05) in sedation scores between the groups and no sedation after 3 hours. Haemodynamic parameters [Table/Fig-8,9].
Duration of absolute analgesia, block and side effects.
| Parameter | Group A | Group B | p-value |
|---|
| Duration of absolute analgesia (hours) after caudal block | 6.53±1.12 | 12±2.22 | <0.0001 |
| Motor blockade | Absent | Absent | NS |
| Number of rescue analgesic doses in 24 hours | | | |
| One dose | 4 | 24 | <0.01 |
| Two doses | 18 | 6 | <0.01 |
| Three doses | 8 | 0 | <0.01 |
| Incidence of side effects | | | |
| 1. Nausea/Vomiting | 4(13.3%) | 3 (10%) | NS |
| 2. Urinary retention, respiratory depression, Motor blockade | 0 | 0 |
*NS-Not significant
Data mentioned are mean with SD, absolute numbers or percentages (%)
Duration of absolute analgesia (hours) after caudal block.
Sedation score in the first 2 hours after surgery.
Mean heart rate and standard deviation.
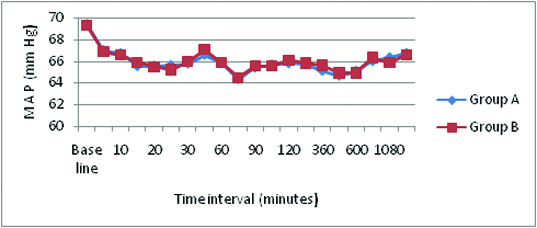
Mean arterial pressure and standard deviation.
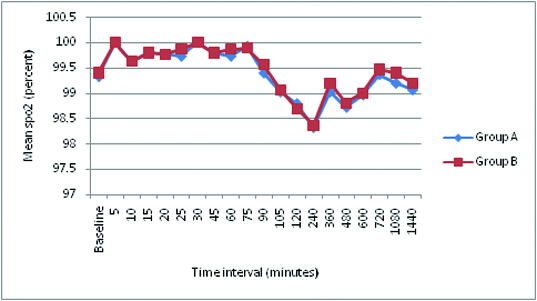
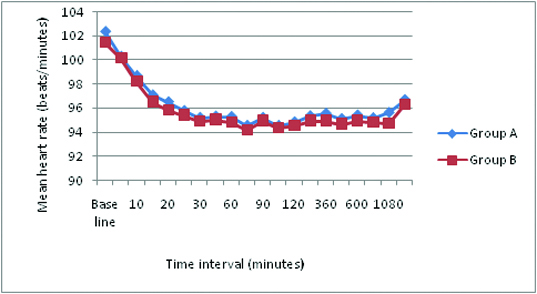
There was no significant difference (p >0.05) in Mean heart rate and mean arterial pressure between the two groups at any time interval. No Bradycardia or Hypotension in both the groups. There was no significant difference (p >0.05) in the oxygen saturation [Table/Fig-10] between the two groups at any time interval. No respiratory depression was observed at any time. There was no incidence of hypotension, bradycardia, urinary retention, motor blockade and respiratory depression in both the groups.
Mean oxygen saturation and standard deviation.
Discussion
In paediatric regional analgesia, single shot caudal epidural technique is one of the most reliable, safe and easy block to administer and is therefore, a commonly performed procedure for intraoperative and postoperative analgesia especially for infra umbilical surgeries in young children. One of the main drawbacks of this technique is the short duration of analgesia [12], even with the use of long acting local anaesthetics like Bupivacaine and Ropivacaine. Ivani G et al., showed 0.2% (2mg/kg) of Ropivacaine (1ml/kg) was enough to provide sensory block for lower abdominal surgeries [13] than 0.25% of ropivacaine which in same dose caused maximal plasma concentration after caudal injection [14] and Bosenberg and colleagues compared the analgesic efficacy of caudal block using different concentrations 1mg, 2mg and 3mg × ml(-1) of Ropivacaine and found that Ropivacaine 2mg ×ml(-1) (1ml/kg) provided satisfactory postoperative analgesia after elective inguinal surgery [15]. They found 1mg ×ml (-1) was less efficacius in providing analgesia and 3mg ×ml (-1) caused more motor blockade which is undesirable. So we chose 0.2% (2mg/ml) Ropivacaine which showed prolonged duration of action.
Ivani G et al., used plain 0.2% Ropivacaine (1ml/kg) and combination of 0.1% ropivacaine and clonidine (2 μg/kg) combination resulted in better analgesia in 0.1% ropivacaine –clonidine group, showed the group of clonidine and Ropivacaine (R 0.1) didn’t require rescue analgesia in first 24 hours [9]. S J Bajwa and colleagues found that the mean duration of analgesia was 8.5 + 3.4 hours with Ropivacaine 0.25% and 13.4 + 3.4 hours with Ropivacaine 0.25% and Clonidine 2 μg/kg without much haemodynamic or respiratory side effects of combined group [8]. Recently Manickam A et al., conducted a study to compare the efficacy of Ropivacaine 0.1% with Clonidine 1 μg/kg to that of plain 0.1% and 0.2% Ropivacaine for caudal analgesia in children and found that analgesia was prolonged in 0.1% ropivacaine with 1 μg/kg combination [16]. Laha A et al., showed addition of 2μg/kg to 0.2% ropivacaine prolonged the analgesic duration to (975±40.5 minutes) compared to only ropivacaine group (466±0.94 minutes) without side effects [17]. Our study correlated well with the above studies regarding analgesia by using 1μ/kg of clonidine and 0.2% ropivacaine (group B) (12±2.22 hours compared to 6.53±1.12 hours for ropivacaine group (Group A), so that group B required lesser rescue analgesica in first 24 hours.
In children, clonidine doses of 1 μg/kg, 1.5μg/kg, 2 μg/kg have been used without any adverse respiratory or haemodynamic effects. However, higher doses 5μg/kg clonidine [18] has been used in combination with lower concentration of bupivacaine were associated with bradycardia and lower blood pressure. In our study we used 1μ/kg of clonidine as an adjuvant to ropivacaine.
Our study required sensory level till T10 dermatome as operative procedures were subumbilical. The prescription scheme of Armitage suggests that for thoracolumbar blockade dose of local anaesthetic to be used is 1ml/kg. In our study, we have used 0.2% Ropivacaine which provides better quality of analgesia when compared to lower concentrations and Clonidine 1μg/kg which prolongs the duration of analgesia significantly and prove it as optimal mixture of drugs to be used which was not used by any previous study where either different concentration of ropivacaine or clonidine was used. Our study showed no significant difference in haemodynamic parameters like Heart Rate (HR), MAP, SBP, DBP and also no respiratory depression (Respiratory depression was defined as oxygen saturation of < 93%) between the two groups which correlated well with study result conducted by SJ Bajwa and colleagues. Pain assessment is the most important and critical component of pain management. Assessing pain in children is an ever challenging as well as a difficult task. In our study, we have used FLACC pain scale [19]. Rescue medication was administered in the form of oral paracetamol syrup 15mg/kg when the FLACC score was ≥ 4. In our study, there was no significant difference in sedation scores between the groups, which correlates well with the other studies [8,9].
Our study showed no residual motor blockade, which was assessed at the end of 2 hour and 6th hour correlated well with other previous studies [8,9,20].
Complications
In our study, 4(13.3%) of the children in Group A and 3(10%) of them in Group B had an episode of vomiting. The incidence of vomiting was comparable in both the groups. The addition of Clonidine to Ropivacaine in our study did not result in increase in the incidence of any other side effects.
Limitations
This is a single centre study which included 30 numbers of patients in each group which might be less and may need more number of studies with same concentration of the drugs. We did not measure plasma concentrations of local anaesthetics during the study.
Conclusion
Combination of Ropivacaine and Clonidine in the concentration used (0.2% ropivacaine and 1 μg/kg of clonidine) can be optimal for postoperative analgesia in paediatric population for infraumbilical surgeries without side effects.
Sedation was assessed by sedation score 0- awake, 1- arousable by voice, 2- arousable to pain, 3- unarousable. Score: 0 = no pain; 1-3 mild pain; 4-7 moderate pain; 8-10 severe pain
Data are mentioned as mean ±standard deviation M-Male F – Female
Data represented absolute numbers (percentages) and p-values at each intervals
*NS-Not significant
Data mentioned are mean with SD, absolute numbers or percentages (%)