Diabetes mellitus is one of the fastest growing metabolic disorders in the world and a major cause of morbidity in developed countries [1]. It is a group of metabolic disorders characterized by hyperglycaemia resulting from defect in insulin secretion, insulin action or both [2]. Worldwide; about 3.2 million diabetes-related deaths are reported annually [3]. In India the increase is estimated to be 58%, from 51 million people in 2010 to 87 million in 2030 [4]. Global morbidity and mortality associated with diabetes is around four million deaths in the age group of 20-79 years in 2010(International Diabetes Federation (IDF) Report 2009) [5].
Diabetes occurs when adipose tissue becomes “full” and fat overflows into organs such as liver, pancreas and muscle, causing insulin resistance. Peroxisome proliferator activated receptor gamma (PPARγ) is required for the development of adipose tissue and control of insulin sensitivity [6]. The peroxisome proliferator-activated receptors are transcription factors belonging to the nuclear hormone receptor superfamily [2].
Protein Kinase C (PKC) is serine-threonine kinases that catalyse various biochemical reactions critical to cellular function. PKCβI and βII isoforms are usually activated in diabetes-related renal and vascular injury, acting via stimulation of multiple signalling pathways [7].
Although many drugs and interventions are available to manage diabetes, these can add to economic burden for the large diabetic population of developing countries like India, apart from their inherent adverse effects. Dietary interventions can play an important role in controlling blood glucose and hence it is necessary to look at alternatives to manage this major health problem.
Materials and Methods
The study was conducted at the Department of Biochemistry, JSS Medical College, Mysore and had the approval from Institutional Animal Ethics Committee with registration number of 261/PO/ReBi/2000/CPCSEA renewed on 14-08-2015 from Government of India. Animals used were adult healthy albino rats of Wistar strain, weighing between 170-250 gm of either sex. The rats were inbred in the central animal house of the JSS Medical College, Mysore, under suitable conditions of housing, temperature, ventilation and nutrition. The animals were fed with commercial laboratory food and water ad libitum. They were maintained at a temperature of 24-27°C with relative humidity of 30-70 % with 12 hour light dark cycle. Following overnight fasting, 18 rats were intraperitoneally injected with freshly prepared streptozotocin (dissolved in sodium citrate buffer) under aseptic precaution in a dose of 55 mg/kg body weight [1] 3 days before the experiment. Blood glucose level was recorded daily morning at around 9.00 am for 3 days. All animals developed stable hyperglycaemia after 3 days. Only those animals with blood glucose level more than 250mg/dl were selected for the study [1].
Animals were randomly divided into four groups, six animals in each group-
Group 1: NORMAL CONTROL: consisted of normal rats which were given 0.5 ml gum acacia.
Group 2: DIABETIC CONTROL: The diabetic rats (STZ 55mg/kg BW, i,p) were treated with 0.5 ml gum acacia.
Group 3: STANDARD GROUP: The diabetic rats (STZ 55mg/kg BW, i,p) were treated with pioglitazone suspended in 0.5 ml of gum acacia, in a dose of 45 mg/kg body weight.
Group 4: TEST GROUP: The diabetic rats (STZ 55mg/kg BW, i,p) were treated with fresh BMJ in a dose of 6 ml/kg body weight.
Bitter melon fruits were purchased from a local shop washed with water, weighed and juice was freshly prepared from the whole fruit in a regular household juicer. Dose of BMJ was 6 ml/kg body weight [10].
Body Weights (BW) of the individual rats were measured on the respective days before blood sugar estimation. Blood was collected from overnight fasted rats after the last dose administration by tail bleeding and blood glucose was estimated by glucose oxidase method on 0, 7, 14, 21 and 28th day. After 28 days, the rats were sacrificed by cervical dislocation and blood was collected from abdominal vena cava in plain vacutainer for estimation of triglycerides by Glycerol 3 phosphate oxidase phenol aminophenazone method and cholesterol by Cholestrol oxidase phenol aminophenazone method. Both the kidneys were dissected out and washed in ice cold Phosphate Buffer Saline (PBS), weighed and stored in PBS at -20°C and used for the analysis of PKC-β and PPAR-γ within 6 months.
Kidneys were rinsed in ice-cold PBS to remove excess blood thoroughly and weighed before homogenization. The kidneys were minced to small pieces and homogenized in 5-10 mL of PBS with a glass homogenizer on ice. The resulting suspension was subjected to two freeze-thaw cycles (-20° C) to further break the cell membranes. After that, the homogenates were centrifuged for 5 minutes at 2000rpm. The supernatant was removed and assayed immediately.
Activities of PKC-β and PPAR-γ were determined by double antibody method Enzyme Linked Immunosorbent Assay (ELISA) using kits from Uscn Life Science Inc. as per the kit manufacturer’s instructions using automated ELISA reader and washer from Bio-rad laboratories, Inc. Performance characteristics of individual tests are as follows: Test for PKC-β had a sensitivity of 0.051ng/ml and PPAR-γ had a sensitivity of 0.058ng/ml with an intra-assay precision of 10%.
Statistical Analysis
Collected data was expressed as Mean±SD. A one-way ANOVA model was used to compare means between groups. All the grouped data were analysed using SPSS version 18 for windows. The differences between means were interpreted statistically significant at p <0.05.
Results
In the present study the effect of fresh BMJ on blood glucose, total cholesterol, triglycerides, PKC-β and PPAR-γ have been evaluated and its efficacy has been compared with that of Pioglitazone in streptozotocin induced diabetic rats.
The control group of rats showed minimal change in blood glucose levels. The diabetic control rats showed consistent hyperglycaemia and the test drug showed persistent decrease in the Blood Glucose Level (BGL) from 1st to 28th day, while the standard drug did not show appreciable decrease in BGL from 1st to 7th day but thereafter produced persistent decrease in BGL upto 28th day.
In control group mean values of blood glucose levels range between 81 on day 0 to 79.5 mg/dl on day 28 without much of variation during the study. In untreated diabetic control rats, the blood glucose levels increased gradually from 341.2mg/ dl on 0 day to 430.5mg/dl on 28th day showing an increase in the blood glucose value. In Pioglitazone treated rats the mean blood glucose level was 370.5 mg/dl on 0 day, which showed decrease only after 7th day, and then steadily decreased to 115.5 mg/dl on the 28th day, here there is persistent reduction of blood glucose level from D1 to D 28.
In Momordica charantia treated group the blood glucose level on day 0 was 363.3 mg/dl, which reduced to 318.5 on day 7 and later there was persistent reduction of blood glucose level from 265.7 on day 14 to 147mg/dl on day 28 which was statistically significant compared to diabetic control [Table/Fig-1].
MEAN±SD values of Blood Glucose levels in mg/dl in different groups.
| GROUPS | D0 | D7 | D14 | D21 | D28 |
|---|
| Control | 81±5.762 | 81±4.733 | 79.83±6.145 | 80.83±5.529 | 79.5±4.183 |
| Diabetic control | 341.2±24.64 | 368+36.57 | 394.5±44.94 | 410.3±49.43 | 430.5±58.45 |
| Standard (Pioglitazone) | 370.5±11.76 | 374±22.77 | 304.7±14.4 | 215.3±11.71 | 115.5±12.8 |
| Test (BMJ) | 363.3±10.67 | 318.5±15.03 | 265.7±14.75 | 220.2±14.08 | 147±17.33 |
The above data shows that there is good hypoglycaemic effect of the herbal drug- Momordica Charantia (6 ml/kg body weight) and is comparable to that of the standard drug Pioglitazone (45 mg/kg body weight).
There was no much change in body weight in control group of rats throughout the study but in diabetic control group, there was reduction of 20% body weight from day 1 to day 28 while in the standard group there was increase in body weight of upto 10% and in the test group there was slight reduction in body weight [Table/Fig-2].
Mean body weight of rats in grams in different groups on different days.
| GROUPS | D0 | D7 | D14 | D21 | D28 |
|---|
| Control | 185±02 | 185±03 | 182±03 | 187±02 | 189±02 |
| Diabetic control | 200±02 | 183±04 | 176±02 | 170±02 | 164±03 |
| Standard | 190±03 | 182±03 | 191±02 | 195±02 | 200±02 |
| Test | 188±02 | 184±03 | 190±04 | 189±02 | 180±02 |
Total cholesterol in test group was 79.6 mg/dl and in standard group it was 87 mg/dl it is decreased in both the groups when compared to diabetic control 109.8 mg/dl and normal control group 93.8 mg/dl. Triglycerides in test group was 86.3mg/dl and in standard group it was 85 mg/dl it is decreased in both the groups when compared to diabetic control 115.5 mg/dl and normal control group 98.5 mg/dl [Table/Fig-3].
Bar graph showing comparison of Total Cholesterol and Trigylcerides between different groups on day 28.
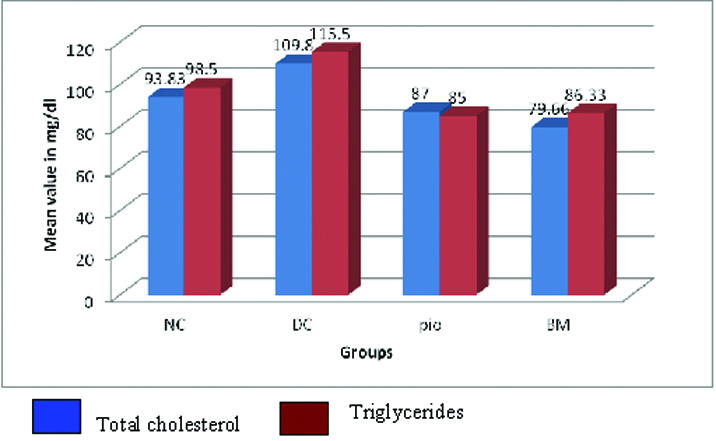
The above data shows that there is good hypolipidemic effect of the herbal drug- Momordica Charantia (6 ml/kg body weight).
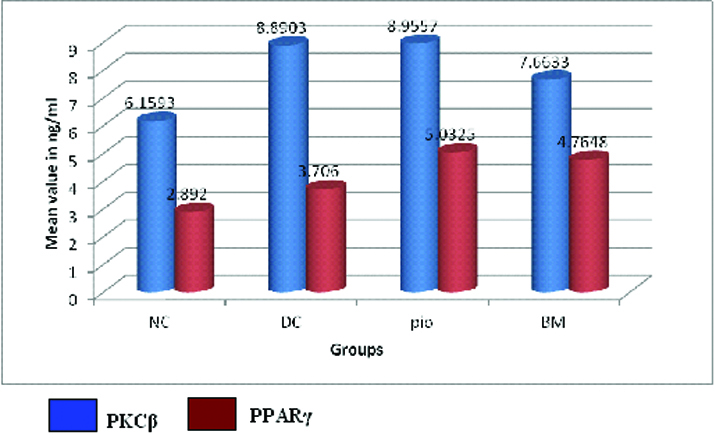
In diabetic control group there was 43% increase in PKC-β activity when compared to normal control. The mean value of PKC-β activity in diabetic control group was 8.890±0.88 ng/ml which was significantly high when compared to test group which was 7.663±0.70 ng/ml. In test group there was 16% decrease in PKC-β when compared to diabetic control. In standard group the mean value of PKC-β was not significant when compared diabetic control group. In test group there was 17% reduction in PKC-β when compared to standard group.
The mean value of PPAR-γ activity in diabetic control group was 3.706±0.57 ng/ml which was significantly low when compared to test group which was 4.764±0.35 ng/ml and standard group 5.032±0.47 ng/ml. There was 28% and 35% increase in PPAR-γ activity in test and standard group when compared to diabetic control. BM and pioglitazone show similar activity on PPAR-γ [Table/Fig-4].
Bar graph showing comparison of PKCβ and PPARγ between different groups on day 28.
Discussion
Hypoglycaemic activity of fresh BMJ is due to the bioactive components, charantin (a steroid glycoside), vicine and polypeptide "p" or plant insulin (a 166 residue insulin mimetic peptide). Mechanisms of action include increased insulin-like effects, stimulation of pancreatic secretion, leading to decreased hepatic gluconeogenesis, increased hepatic glycogen synthesis and increased peripheral glucose oxidation [8].
Fresh BMJ has good hypolipidemic effect. Momordica charantia increases the activity of adenosine 5 monophosphate kinase (AMPK), an enzyme that regulates cellular glucose uptake and fatty acid oxidation. They decrease liver secretion of apolipoprotein B (Apo B) – the primary lipoprotein of low-density cholesterol; also reduces apolipoprotein C- III expression, the protein found in very-low density cholesterol which turns into LDL/bad cholesterol; and increases the expression of apolipoproteinA-1 (ApoA1) - the major protein component of high density "good" cholesterol. It also decreases cellular triglyceride [11].
Different studies have tried to study the oral hypoglycaemic and hypolipidemic effects of Momordica charantia using various parts of the plant. Studies using oral administration of aqueous extract by Bano F et al., over a period of five weeks showed significant decrease in blood glucose (17%, p<0.01), cholesterol (21% p<0.01), triglyceride (20% p<0.01), LDL cholesterol (20% p<0.01) and increase in HDL (45% p<0.05) [11]. Similarly Fernandes N et al., have shown that oral administration of Momordica Charantia extract showed significant reduction in cholesterol, triglyceride, LDL-CH, VLDL-CH and blood glucose levels as compared to untreated diabetic rats [12]. In another study conducted by Chaturvedi et al., it has been shown that administration of methanol extract from dried fruits significantly decreased triglyceride and LDL and increase in HDL (p<0.001) [13].
The body weight of diabetic control rats were decreased whereas the body weight gradually increased in pioglitazone treated rats and slight reduction in bitter melon treated group.
Though most of the studies have tried to study the glucose and lipid lowering action of MC, the mechanisms by which they are attained still remains largely obscure and the focus of recent studies has been the same [14]. Since PPAR is a key modulator of adipogenesis and lipolysis there have been studies which have studied the effects of MC extract on PPAR [15]. Similarly PKC is implicated in the inflammatory pathway triggered due to hyperglycaemia and has been thought to be involved in the complications arising due to DM.
PPARγ is present in adipocytes and is a master regulator of adipogenesis and invivo, as an insulin sensitizer, as evidenced by the effects of TZDs as PPARγ agonists [16].
Recent studies have showed PPARγ is involved in the normal kidney development, renal lipid metabolism and activation of the renin-angiotensin system [17]. Synthetic PPARγ ligands can ameliorate the diabetic kidney disease through various mechanisms, involving inhibition of mesangial cell growth, reduction of mesangial matrix, and cytokine production of glomerular cells as well as promoting endothelial cell survival within the kidney glomeruli collecting system [18].
Different studies have been done to see the expression of PPAR-γ in diabetic rats and their involvement in diabetic complications. Yang H et al., showed that renal PPAR-γ activity was significantly increased in the pioglitazone group compared with control and also showed a decreased mitochondrial injury through reduced protein kinase C-β and p66Shc phosphorylation. The aging-related renal injury may be benefited by the novel effects of PPAR-γ agonist by improving the function of the mitochondria [19]. Guo B et al., showed PPAR-γ could inhibit TGF-β signalling cascades via the interaction of the AP-1complex and PPAR-γ strengthens the beneficial role of TZD in treating fibrotic kidney disease, including diabetic nephropathy [20].
In the current study, the activity of PPAR-γ when compared to the diabetic control group was significantly increased in both the test and standard group. This confirms that BM like pioglitazone acts as PPAR- γ agonist. Hyperglycaemia is one of the major causes of diabetic vascular complications involving both microvessels and arteries in the retina, renal glomeruli and aorta. In diabetic nephropathy, an increase in DAG and PKC-β isoforms leads to dysfunctions in the micro and macrovasculature of the kidney and progressive chronic kidney disease. The ability of isoform-specific PKC inhibitors to antagonize the development and progression of these diseases is providing new avenues for their treatment [7]. Patrica A et al., have showed an increase in PKC-β activity in the glomeruli of diabetic rats [21]. Inoguchi T et al., showed an increase in PKC- β in aorta and heart of STZ induced diabetic rats [22].
In our study the activity of PKC-β activity was significantly reduced in test group when compared to diabetic control group thus fresh BMJ decreases the PKC-β thereby preventing the diabetic vascular complications involving both microvessels and arteries in the retina, renal glomeruli and aorta.
Though the debate over which isoform of PKC is primarily responsible for complications related to hyperglycaemia continues, there a number of studies which have mainly studied the isoform β and have tried inhibitors against the PKC β isoform producing favourable results in vitro. But search for interventional studies with drugs or plant extracts have on different databases did not yield any results and many of the interventional studies are still in phase II clinical trials.
Limitation
The present study has several limitations. The study has been carried out only in one species of animal’s viz., —rats and may be extended to other non rodents as well. In the present study we have used single dose of 6 ml/kg BW as a testing dose. Further studies need to be done to fix proper dosage. Acute and chronic toxicity testing need to be undertaken.
Conclusion
The results indicate that the test compound Momordica Charantia at a dose of 6 ml/kg BW has significant and sustained oral hypoglycaemic and hypolipidemic activity in streptozotocin induced diabetic rats. The test drug however showed an immediate onset of action which was long acting and needs to be further evaluated before the compound could be used as an adjuvant to standard hypoglycaemic agents for better glycaemic control or as monotherapy in mild to moderate cases. BMJ increases PPAR-γ activity and decreases PKC-β activity in glomeruli of diabetic rats thereby preventing the complications of diabetes mellitus. Fresh BMJ mimics action of pioglitazone belonging to TZD group thus showing a potential for further research in identifying the active molecules responsible for glucose and lipid lowering action. Further studies in this respect along with long term safety studies and clinical trials are necessary to add this novel drug to the existing ones for overall management of type2 DM.