Dyslipidemia has emerged as a growing health concern as it constitutes one of the major risk factors for the development of cardiovascular diseases, such as atherosclerosis and its complications like myocardial infarction, cerebral infarction, aortic aneurysms and peripheral vascular disease [1,2]. The dose dependent hepatotoxicity and myopathy associated with the currently used treatment modalities [3,4] have resulted in a new impetus for the search of better tolerated agents.
Although, NFJ has been traditionally used for several ailments, scientific evidence supporting its nutritional and medicinal values in humans is limited. Except for a survey report [5], published data on the beneficial effects of Noni in humans is lacking.
The Noni commercial preparations are marketed in India under the title ‘natural food supplement’ claiming several health benefits to lure the patients. The lipid lowering and anti-obesity potentials of NFJ have been claimed by noni manufacturers, the scientific evidence for which is yet to be established [10,11]. In an in-vitro bioassay using rat HMG-CoA reductase enzyme, that is involved in the synthesis of cholesterol, different concentrations of NFJ inhibited the enzyme in a dose dependent fashion [12,13]. Studies done in vitro, in animals and in current smokers demonstrated the protective effect of NFJ on superoxide anion radicals and lipid peroxides [5]. These actions of NFJ may contribute to lowering lipid levels. A double–blind placebo controlled study, carried out in small population of current smokers demonstrated a decline in TC and TG levels in NFJ drinkers, the drop being greater in patients with higher pre-treatment values [13]. Hence, the lipid lowering benefits of NFJ still needs to be verified and established.
The present study was designed to evaluate the effects of NFJ on serum lipid profile in high fat diet induced dyslipidemic rat model.
Materials and Methods
Animals
Male wistar rats weighing 150g-200g were obtained from central animal house of our institution. Rats were housed in clean polypropylene cages; three rats were placed in each cage in a controlled environment (26°-28°C) with a 12 hour light/dark cycle. Standard chow (containing fat 4.15%, protein 22.15%, carbohydrates 4% manufactured by Pranav Agro industries Ltd., Sangli) and water was provided ad libitum. The rats were allowed to acclimatize to these conditions for one week. The study was conducted after obtaining the permission from the Institutional animal ethics committee.
Chemicals
Pure cholesterol powder was purchased from Himedia chemicals, Mumbai. Deoxycholic acid was purchased from Sigma Aldrich chemicals, Bangalore. Atorvastatin was obtained from Ranbaxy Laboratories Ltd. India.
Study Drug
Drip extracted NFJ preparation supplied by Alwa’s Ayurvedic Pharmacy, Moodbidre, and Mangalore was used for the study. The concentration of NFJ used was 5g/dL (5%).
Acute Toxicity Study [
14]
Acute toxicity was studied with oral administration of NFJ, using the OECD 2006 guidelines [15]. As per the limit test, female wistar albino rats (150-200g) were fasted overnight and given 2000 mg/kg of NFJ orally, the next day. Animals were observed for 48 hours, with special attention during the first 4 hours for any abnormal behavior such as sedation, respiratory distress, motor impairment, and hyper-excitability; thereafter, for a period of 14 days for any signs of toxicity or mortality. Likewise, five animals were dosed and observed one followed by the other. The juice was found safe even at a dose as high as 2000 mg/kg without any sign of toxicity or mortality. Hence, the dose of NFJ (50mg/kg and 100mg/kg) used in the study were considered safe.
Preparation of High Fat Diet
Deoxycholic acid (5g) was mixed thoroughly with 700g of powdered rat chow. Simultaneously, cholesterol (5g) was dissolved in 300g warm coconut oil. The oil and cholesterol mixture was added slowly into the powdered mixture to obtain a soft homogenous cake. This cholesterol-rich High-Fat Diet (HFD) was molded into pellets of about three gram each [6].
Study Procedure
Rats were randomly assigned to five groups of eight rats each. The feeding and treatment schedule was as follows. Group 1 received only normal chow whereas all the other groups received HFD. Group 3 received atorvastatin; group 4 and group 5 received the NFJ via oral gavage along with HFD.
Group 1: 2ml of 0.1% w/v tween 80 (Normal control).
Group 2: HFD (Dyslipidemic control).
Group 3: HFD + atorvastatin 10mg/kg bd. wt. was given as a suspension in 0.1% w/v tween 80.
Group 4: NFJ 50 mg/kg bd. wt. + HFD.
Group 5: NFJ 100 mg/kg bd. wt. + HFD.
Body weight was recorded at the beginning i.e. the day 1 of treatment and at the end of the drug administration (day 31) in each group. Three readings of the weight were recorded each time the animal was weighed and a mean value of these three readings was taken.
On day 31, rats were anesthetized with sodium pentobarbitone (40mg/kg) given intraperitonially and 2ml of blood was drawn by an intra-cardiac puncture. The blood sample was then centrifuged at 3000 rpm for 10 minutes and clear serum was aspirated, stored, frozen and then utilized for assessment of lipid profile using a Hitachi 902 biochemistry analyser. Following this, the rats were euthanized by intaperitonial injection of sodium pentobarbitone (100mg/kg). Liver was dissected out, cleared of blood and collected in 10% formalin solution. These tissues were processed and embedded in paraffin wax block and thin new microtome serials (2-3 μm thick) were cut and stained with Haemotoxylin and Eosin (H&E) stain. Fatty changes or necrotic changes if any in each group were noted and compared with the control group.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) 17.0 version was used for the analysis of the data. The p-value of less than 0.05 was considered as significant. For analysis of the differences in the biochemical measures (lipid profile) between the five groups, one-way ANOVA (Analysis of Variance) was used. This was followed up with Tukey post-hoc test. For comparing the body weights of rats on day 0 and day 31, paired t-test was used. In order to analyze the differences in the weight gain over a period of 30 days one-way ANOVA followed by Tukey post-hoc test was applied.
Results
Lipid Profile
As detailed in [Table/Fig-1], administration of HFD significantly increased the TC, TG, HDL-C, LDL-C and VLDL-C levels (p<0.05) in the disease control (Group 2) when compared to normal rats. The standard drug atorvastatin decreased all plasma lipids but statistical significance was observed only with total cholesterol (107.25±5.99 mg/dL) and LDL-C (43.82±6.15 mg/dL) when compared to the disease control.
Effect of NFJ on plasma lipids in high fat diet induced hyperlipidemic rats. All the values are expressed as mean ± SD
| Group | Total cholesterol(mg/dL) | Triglycerides(mg/dL) | HDL-C(mg/dL) | LDL-C(mg/dL) | VLDL-C(mg/dL) |
|---|
| 1 | 71.13±3.52 | 82.75±17.91 | 22.15±1.35 | 32.05±5.75 | 16.95±3.04 |
| 2 | 127.75±8.13* | 184.50±15.26* | 31.38±8.46* | 56.77±6.04* | 36.47±2.86* |
| 3 | 107.25±5.99*a | 167.38±13.76* | 30.13±2.47* | 43.82±6.15*a | 33.30±2.88* |
| 4 | 102.75±9.79*a | 90.00±12.05ab | 36.87±2.58*b | 47.87±7.47* | 18.0±2.41ab |
| 5 | 109.75±11.27*a | 86.00±7.36ab | 36.63±3.11*b | 56.05±9.39* | 17.20±1.47ab |
* - p<0.05 compared to control (Group 1); a - p<0.05 compared to disease control (Group 2); b - p<0.05 compared to standard drug group (Group 3)
The NFJ treated group showed a significant decrease in the TC, TG and VLDL-C at both the doses when compared to the disease control. However, the observations did not reflect a dose dependent decrease. The decrease in the total cholesterol (102.75±9.79 mg/dL) and Low Density Lipoprotein-Cholesterol (47.87±7.47 mg/dL) levels observed with the NFJ at the 50mg/kg dose employed, failed to show a statistical significance when compared to atorvastatin. A similar observation was also noted with LDL-C levels (56.05±9.39 mg/dL) in rats treated with NFJ at the dose of 100mg/kg.
The HDL-C levels were significantly increased in all the groups receiving HFD. The increase noted were comparably similar at both the doses of NFJ employed, signifying no dose dependent action on HDL-C.
Changes In Body Weight
There was a significant (p<0.05) gain in body weight in all the groups over a period of 30 days. The increase in body weight was higher in the group fed with only high fat diet i.e. group 2 (91.83±24.02g) compared to the rest of the groups. Weight gain was least in the normal control group (27.67±8.96g). The weight gain in the standard drug group (group 3) was not significant in comparison with normal control as well as the test groups (group 4). Weight reduction was better with NFJ (50mg/kg) in comparison with NFJ (100mg/kg) but the differences were not statistically significant. The weight reduction in group 4 (58.50±15.83g) was significant (p<0.05) when compared to disease control (91.83±24.02g) as shown in [Table/Fig-2].
Effect of NFJ on body weight in high fat diet induced hyperlipidemic rats.
| Groups | InitialBody Wt. (g) | FinalBody Wt. (g) | Change in Body Wt. (g) |
|---|
| 1 | 204.17±6.24 | 231.83±14.39 | 27.67±8.96 |
| 2 | 202.17±11.43 | 294.00±21.34 | 91.83±24.02* |
| 3 | 208.00±7.69 | 262.67±8.52 | 54.67±9.17X |
| 4 | 203.00±14.64 | 261.50±14.12 | 58.50±15.83*X |
| 5 | 192.00±19.67 | 263.17±10.03 | 71.17±20.95* |
All values expressed as Mean ± SD
* denotes p<0.05 control group (group 1) compared to all other groups fed on high fat diet
x denotes p<0.05 when group 2 is compared to rest
Histopathological Evaluation
As evident from [Table/Fig-3], the histological alterations such as steatosis, sinusoidal dilatation and congestion were not present in normal control group. Rats fed only HFD (Group 2) showed microvascular steatosis and occasional macrovascular steatosis of hepatic lobules [Table/Fig-4] along with sinusoidal dilatation and mild congestion. The portal trial revealed increase in inflammatory infiltrate of mononuclear cells and neutrophils. In the standard control group normal lobular architecture was maintained and no fatty change was seen [Table/Fig-5]. In both the test groups (Group 4 and Group 5), the normal lobular architecture was maintained, chronic inflammatory infiltrate was seen along with marked congestion. Moderate fatty change in the hepatocytes was seen in NFJ 100mg/kg group (Group 5) as shown in [Table/Fig-6,7].
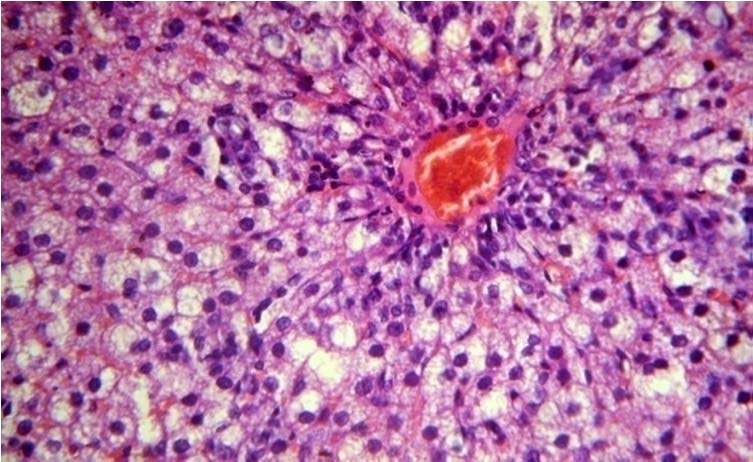
Normal histology (Group 1).
Normal hepatocytes are arranged in anastomosing sheets and plates around the central vein. Normal hepatocytes showing round nucleus with granular eosinophilic cytoplasm. (40X, H&E)
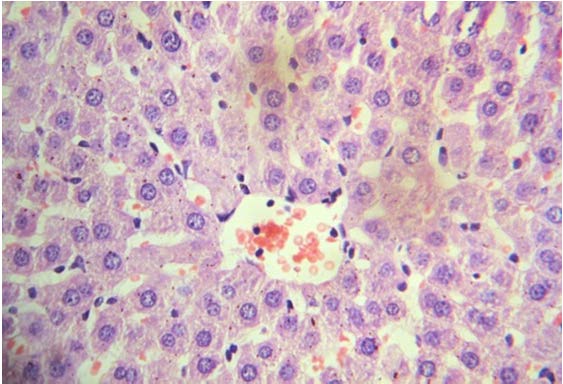
Hyperlipidemic group (Group 2).
Loss of normal lobular architecture. Hepatocytes show numerous small vacuolation (microvesicles) in cytoplasm [black arrow] and in few the nucleus is pushed to the periphery of the cell giving a signet ring appearance (macrovesicles). (40X, H&E).
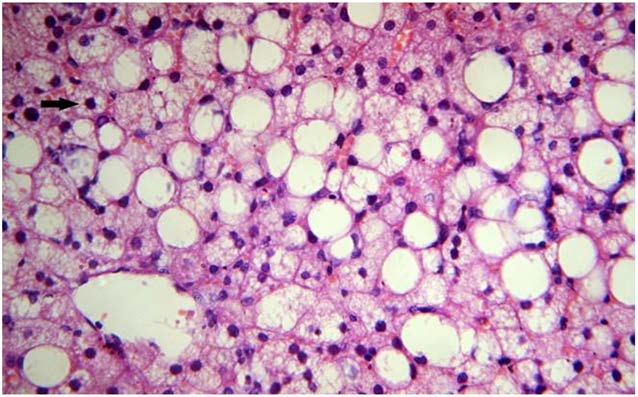
Standard control (atorvastatin) (Group 3).
Normal lobular architecture preserved. Normal hepatocytes showing mild anisonucleosis and fine granular cytoplasm. No fatty change seen (40X, H&E)
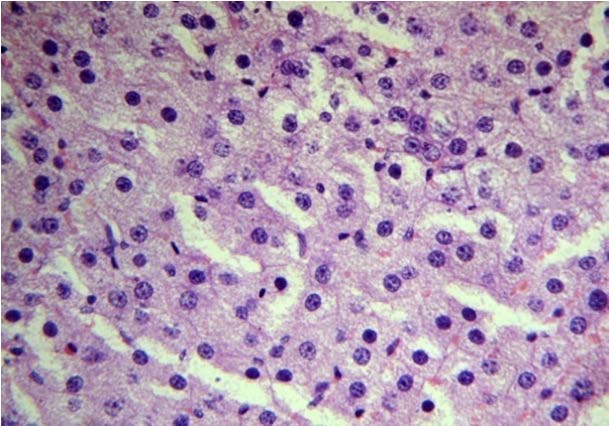
Test group I (NFJ 50mg/kg bd. wt) (Group 4).
Marked congestion surrounding the hepatoctes. (40X, H&E)
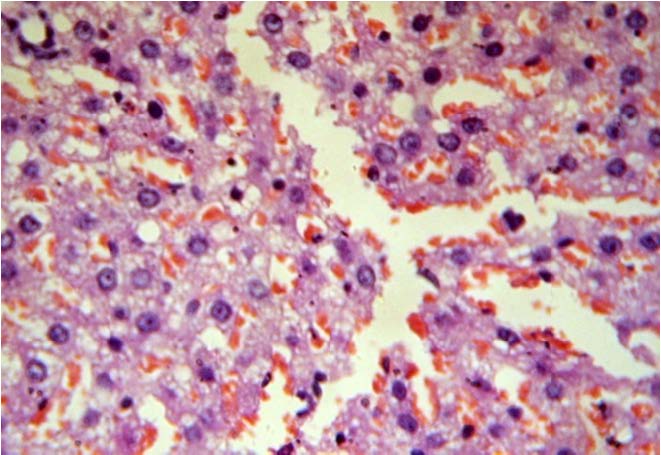
Test Group II (NFJ 100 mg/kg bd. wt.)
Hepatocytes surrounding the central vein show moderate fatty change microvesicular and macrovesicular. (40X, H&E)
Discussion
The current study was undertaken to evaluate the hypolipidemic activity of NFJ in HFD induced hyperlipidemic rats, as testimonials claiming health benefits of NFJ are abundant on countless internet sites but data published in scientific literature are really scant.
In the present study, hyperlipidemia in rats was well established by feeding them with HFD which consisted of 5g cholesterol, 5 g deoxycholic acid, 700g rat chow powder and 300g of warm coconut oil [16]. A significant increase in all the lipoproteins as well as triglyceride levels was observed as evidenced in [Table/Fig-1]. However, it was also observed that there was an increase in the HDL-C levels in all the animals fed with HFD which could most probably be due to the coconut oil component of HFD. Literature search revealed that prior studies have concluded that coconut oil raises HDL-C levels in rodents [17], non-human primates [18] and also in humans [19]. This is attributed to the high content of lauric acid in coconut oil which is known to increase both total cholesterol and HDL-C [20]. Moreover, coconut oil is also a known antioxidant. This could have contributed to the rise in HDL-C observed in our study.
Noni fruit has been considered useful in cardio vascular diseases particularly atherosclerosis and dyslipidemia [21]. In our study, we used an animal model to evaluate the effects of NFJ on lipid profile in high fat diet induced hyperlipidemia. Treatment with NFJ caused a significant decrease in mean serum total cholesterol, TG, LDL-C and VLDL-C, while it increased HDL-C. These changes in serum lipids were seen with both the doses of NFJ (50mg/kg bd. wt. and 100mg/kg bd. wt.) and there was no significant difference between the effects of the two different doses.
The results of the current study are concordant with a previous study which evaluated the antidyslipidemic effects of ethanolic extract of noni fruit, leaves and roots in high fat diet induced hyperlipidemia [21]. In this study by Mandhukahal SR et al., all the three extracts caused significant reduction in total cholesterol, triglyceride, low density lipoprotein-cholesterol (LDL-C), atherogenic index and TC/HDL ratio [21]. However, the dose used in this study was 1000mg/kg and the treatment with the fruit extract had no significant effect (p > 0.05) on the HDL-C levels. But in the present study a significant increase was observed in HDL-C levels as compared to the control which could be contributed by the presence of coconut oil as a component of the high fat diet induced as explained above. It is well known that increase in HDL-C levels has a protective role in coronary artery disease [22].
In the study conducted by Mandhukahal SR et al., there was no effect seen on body weight and daily diet consumption (p > 0.05) on administration of fruit extract [21]. However, a significant increase in the gain in average body weight and increase in daily diet consumption was observed in the animals fed with the leaf and root extracts. In the present study, there was gain in weight seen in all the groups over a period of 30 days. Maximum gain was seen in the group fed only HFD. The reduction in weight was significant in the lower dose of NFJ, which could contribute to the cardio protective effect of NFJ. However, the higher dose of NFJ did not reduce the weight significantly. Hence, additional studies are required to comment on the long-term effect of NFJ on weight.
An earlier study comparing the hypolipidemic effects of Morinda citrifolia in Tyloxapol a non-ionic surfactant induced hyperlipidemia model has postulated that HMG CO-A inhibition to be the most plausible mechanism of action of the lipid lowering effect of noni. The authors have opined that Tyloxapol increases serum triglycerides and cholesterol levels by increasing hepatic cholesterol synthesis in particular by increasing HMG Co-A (3-hydroxy-3-methyl-glutaryl Co-A) activity. It also inhibits lipoprotein lipase which is responsible for hydrolysis of plasma lipids [21]. An earlier study has also reported the inhibitory effect of noni on HMG CoA activity invitro [23] and hence this could be one of the factors contributing to its action. However, failure of the Noni extracts to cause complete inhibition in earlier studies indicates the involvement of additional mechanisms.
Morinda citrifolia is reported to be rich in flavones, which are known to inhibit lipid biosynthesis [24]. High cholesterol diet induces endothelial dysfunction, atherosclerosis and increases oxidative stress by increasing the expression of oxidation-sensitive genes, such as Elk-1 and p-CREB [21].
Oxidative stress (enzymatic and non-enzymatic) induced by HFD in rats increases oxidation of low density lipoprotein (LDL) which plays a key role in atherogenesis. Strong antioxidant action of NFJ attributed to active constituents like 3, 3’-bisdemethylpinoresinol, americanol A, morindolin, isoprincepin, scopoletin, kaempferol, ursolic acid and quercetin may offer additional benefit in combating the oxidative stress caused by high cholesterol [21]. Various extracts of noni fruit have also demonstrated inhibition of copper induced LDL oxidation which might also contribute to the cardio protective effect of NFJ [25].
The present study provides evidence for the hypolipidemic activity of NFJ. This activity could be due to various mechanisms, inhibition of lipid biosynthesis being one of them. The presence of potent antioxidant activity of NFJ could also contribute to this effect. Furthermore, contribution of other unknown mechanisms cannot be ruled out. Supplementary studies would be required to isolate and pinpoint the active metabolite or metabolites, which produce this effect. Preliminary clinical studies have started to shed more light on the beneficial actions of noni fruit on lipid levels but large scale randomized clinical studies are warranted to establish its safety and efficacy profiles [26].
Conclusion
To conclude, the results from this study rationalize the medicinal use of NFJ in dyslipidemia; however, additional studies especially for a longer duration are required to prove the safety and efficacy of NFJ and its constituents in actual clinical settings.