Collapsing Glomerulopathy (CG) was first described by Weiss et al., as distinct entity with progressive renal failure and pathological changes characterized by segmental or global capillary collapse and visceral epithelial cell hypertrophy and hyperplasia with hyaline droplets and extensive tubular interstitial inflammation [1]. Clinically CG is more common in black race, in severe nephrotic syndrome, with poor response to empirical therapy and rapidly progresses to End Stage Renal Disease (ESRD) [2,3]. CG was known to be associated with Human Immunodeficiency Virus (HIV) infection. However, later on it was also observed in HIV negative patients and hence the term “idiopathic CG” came in to vogue for which the credit goes to Detwiler et al., who were the first to report CG in HIV negative patients [4]. Most of the studies of CG are published from western countries and few from India and Pakistan [2,3,5–8]. We carried out this single center retrospective study to evaluate clinicopathological features and prognosis of idiopathic CG in our set-up. CG is also known to recur or denovo in renal allograft. Our experience of stem cell therapy in renal transplant patients who were subjected to intrathymic stem cell infusion has showed absence of recurrence of FSGS including CG [9]. Perhaps such a study of stem cell therapy for management of CG will throw more light on future of this entity. Similarly role of plasmapheresis and Rituximab in CG can be explored.
Materials and Methods
A total of 3335 renal biopsies were retrospectively analyzed which were performed in our center from January 2008 to December 2014. Inadequate biopsies were excluded from study. All biopsies were performed by Nephrologist under ultrasound guidance using 18 gauge renal biopsy needle. Two cores of renal tissue were taken, one for light microscopy and one for immunofluorescence (IF) studies. Electron microscopy was not performed due to its non-availability. For light microscopy, 3 μm thick paraffin sections were stained for haematoxylin and eosin (H and E), Periodic Acid Schiff (PAS), Jone’smethaneamine silver (JMS) and Gomori’s trichrome (GT) stains. IF sections were stained using anti-human IgG, IgA, IgM, C3, C1q and fibrinogen antisera (MP Biomedical, France). Tests for antinuclear antibody (ANA), anti-double-stranded deoxyribonucleic acid (dsDNA), anti-neutrophil cytoplasmic antibodies {by Enzyme-linked immunosorbent assays (ELISA)}, complement components (C3 and C4) were recorded. ELISA for HIV and hepatitis B and C viruses’ was also carried out.
Demographic evaluation included age, gender, disease duration, hypertension, serum creatinine (mg/dL), 24 hours urinary protein (grams/24 hours) and urinalysis. Hypertension was defined as blood pressure >140/90 mmHg and/or ongoing anti-hypertensive medication. NS was defined as edema, nephrotic range proteinuria (>40 mg/m2/h on timed sample, spot albumin to creatinine ratio >2 mg/dl) and hypoalbuminaemia (>2.5 g/dL). Complete remission was defined as urine protein:creatinine ratio (uPCR) of 200 mg/g (o20 mg/mmol) or o1+ of protein on urine dipstick for 3 consecutive days. Partial remission was defined as proteinuria reduction of 50% or greater from the presenting value and absolute uPCR between 200 and 2000 mg/g (20–200 mg/mmol).
CG was diagnosed morphologically by demonstration of at least one glomerulus with segmental or global capillary collapse with hyperplasia and hypertrophy of visceral epithelial cells and severe tubular interstitial injury. Total number of glomeruli with percentage of globally/segmentally collapsed capillary tufts was reported. Associated involvement of tubulointerstitial compartment in the form of active interstitial inflammation/fibrosis, tubular atrophy and microcystic dilatation were reported as percentage of cortical area involved. Tubular atrophy was graded as t1, t2 and t3 for ≤ 25%, ≤ 50% and > 50% of tubules involved. Similarly interstitial fibrosis was graded as i1, i2 and i3 for ≤ 25%, ≤ 50% and > 50% of interstitium involved.
Statistical Analysis
Correlation of histological findings with clinical and biochemical parameters was carried out. Analysis was performed using IBM SPSS 20. Continuous data were expressed as mean ± SD and non-continuous data were expressed in percentage and numerical values.
Results
A total of 3335 native renal biopsies performed in seven years were evaluated. Twenty five (0.75%) biopsies qualified for idiopathic CG, out of which 22(88%) belonged to adults and 3(12%) belonged to children (≤ 16 years). Sixteen (64%) out of 25 biopsies belonged to females.
The mean age was 36.25±21.25 years in adults and 7.33 ± 3.51years in children. The duration of the symptoms at the time of biopsy was 34.12±26.09 days in adults and 35±22.91 days in children. Hypertension was noted in 9(40.9%) and oliguria in 8(36.4%) adult patients and 1 out of 3 children had oliguria. No hypertension was observed in children. Urinalysis revealed microscopic haematuria in 12(54.5%) adults only. Nephrotic range proteinuria was found in adults only with incidence of 45.5%. The mean 24 hours urinary protein excretion was 7.72±5.66 grams in adults and 2.25±0.46 grams in children [Table/Fig-1].
Demographics with clinical features
| Adult:22 | Children:3 |
|---|
| Age (years) | 36.25±21.25 | 7.33±3.51 |
| Male:Female | 7:15 | 2:01 |
| Duration (days) | 34.12±26.09 | 35±22.91 |
| Hypertension | 9(40.9%) | 0 |
| Haematuria | 12(54.5%) | 0 |
| Oliguria | 8(36.4) | 1(33.3) |
| Serum creatinine (mg/dl) | 3.93±3.06 | 2.46±1.3 |
| 24Hours protein (gm/day) | 7.72±5.66 | 2.25±0.46 |
| Nephrotic syndrome | 10(45.5) | 0 |
The renal biopsies were adequate with mean number of glomeruli being 13.92±5.46 and mean 4.54 ± 3.11glomeruli revealed global/segmental capillary collapse [Table/Fig-2a&b]. There was associated global sclerosis in 7.6±3.72 (30%) glomeruli and segmental sclerosis in 5±3.01 (20%) glomeruli. Tubular microcystic dilatation was seen in 16(64%) biopsies [Table/Fig-2c]. Tubular atrophy was graded as t1 in 15(60%), t2 in 4(16%) and t3 in 6(24%) biopsies. Interstitial fibrosis was noted as i1 in 17(68%), i2 in 2(8%) and i3 in 6(24%) biopsies. Interstitial mononuclear cellular infiltration was seen in all cases [Table/Fig-3]. Immunofluorescence (IF) studies showed non-specific mesangial deposits of IgM± C3 in All biopsies.
(a) Glomerular collapse with hyperplasia/ hypertrophy of podocytes (). (Periodic acid Schiff, x200). (b) Glomerular collapse with hyperplasia/ hypertrophy of podocytes. () (Jone’s silver methenamine, x 400. (c) Microcystic dilatation of tubules () (Gomori’s trichrome (GMT) x 100).
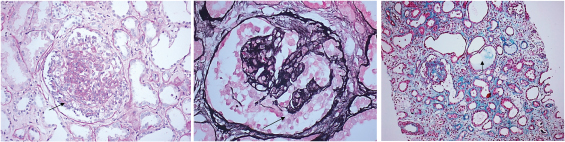
Histopathological findings of renal biopsies
| Number of total Glomeruli | 13.92±5.46 |
| Number of Collapsed glomeruli | 4.54±3.11 |
| GloballySclerosed glomeruli | 7.6±3.72 |
| Segmentallysclerosed glomeruli | 5±3.01 |
| Interstitial Fibrosis |
| Severe >50% i3 | 6(24%) |
| Moderate 26-50%- i2 | 2(8%) |
| Mild ≤ 25%- i1 | 17(68%) |
| Tubular Atrophy |
| Severe >50%- t3 | 6(24%) |
| Moderate 26-50%- t2 | 4(16%) |
| Mild ≤ 25%- t1 | 15(60%) |
| Microcystic dilatation of tubules | 16(64%) |
Ten (40%) patients were lost for follow-up. In remaining 15 (60%) patients with mean follow-up of 19.7±14.6 months, all are on steroids and 7 out of 15 also received calcineurin inhibitors (cyclosporine three patients and Tacrolimus four patients) in addition. Nine (60%) patients have progressed to end stage renal disease and are on maintenance haemodialysis. Histology revealed chronic changes of tubular atrophy and interstitial fibrosis of grade t3 i3 in 4, t2 i2 in 2 and t2 i1 in 3 biopsies. Five (33.3%) patients with chronic changes of grade t1 i1 have maintained stable renal function with mean SCr of 2.6 ± 2.05 mg/dL. Proteinuria declined in five patients with complete remission in two and partial remission in three patients.
Discussion
CG is now a well-recognized distinct morphological pattern of proliferative parenchymal injury which is a harbinger of poor response to empirical therapy [10]. CG was reported to be associated with human immunodeficiency virus associated nephropathy (HIVAN) [11]. Detwiler et al., had reported CG in HIV negative patients [4]. A recently proposed taxonomy for podocytopathies classifies CG apart from FSGS and recognizes three major categories of CG; idiopathic, genetic and secondary or reactive [12]. Black race and COQ2 mutation with mitochondrial dysfunction suggest genetic susceptibility [10]. In addition to HIV infection, parvovirus B19 infection and treatment with Pamidronate are also known to cause CG.
In our study the incidence of CG was 0.75% which was similar to findings of other studies [4,6,8,13,14]. Female predominance was found in our studies similar to report by Laurinavicius [13]. However Ahuja et al., have reported male predominance [6]. The mean duration of biopsy in our study was 35±22.91 days which was comparatively lower than other studies [6,13,15]. Other clinical features like hypertension and oliguria were also comparable with findings of Ahuja et al., [6]. We have observed microscopic haematuria in 12 (54.5%) adults. However, Ahuja et al., have reported haematuria in all their cases [6].
We tried to compare adult CG with childhood onset CG. However since the number of children affected by CG was small (three) in comparison to adults with CG, the comparative evaluation was abandoned.
On histology evaluation, our study revealed about 18% glomeruli showed partial or global collapsed glomerular tufts. Microcytic dilatation was comparably more in our study than study of Ahuja et al., [6].
Remission was observed in 6 patients who presented with serum creatinine < 2mg/dl and chronic changes of tubular atrophy/ interstitial fibrosis of grade t1 i1 whereas with more intense injury and higher serum creatinine, there was limited recovery. Similar observation was also reported by other authors [3,13,16]. However, ESRD was not correlated with age and gender.
The pathogenesis of CG involves visceral cell injury leading to cell cycle dysregulation and proliferative phenotype. Loss of maturity markers like podocalyxin, synaptopodin and loss of WT1 along with re-expression of early podocyte marker (PAX2 and cytokeratin) along with proliferation marker KI-67, indicates dysregulation of phenotype.
Limitation
We could not compare adult CG with CG in children due to unequal data and small sample size of pediatric biopsies. However interesting observation of CG in children emerges that CG was encountered in this age group inspite of absence of haematuria and hypertension. We could not correlate the histology with extent of hypertension and serum creatinine. Perhaps studies correlating renal damage in terms of CG with interstitial fibrosis, tubular atrophy and clinical extent of hypertension and serum creatinine may prove useful. The present study has main focus on histology findings, future studies including clinical management will be helpful.
Conclusion
Idiopathic CG is a morphological pattern of grave podocyte injury with poor prognosis. However there are chances of remission/recovery if the tubular atrophy and interstitial fibrosis are of grades ≤ t1 i1.