Elimination of bacteria during root canal treatment by instrumentation, irrigation and intracanal medication has always been an important part of successful endodontic treatment [1]. Of these three, disinfection of the root canal is a major determinant in the healing of the periapical tissues [2].
Irrespective of thorough cleaning and shaping and intracanal medication, it is difficult to completely eradicate all microorganisms from the canal space, which will further result in endodontic failure [3]. Enterococcus faecalis, is a gram-positive facultative anaerobic bacteria, which have been frequently found in filled root canals with signs of chronic apical periodontitis [4]. When established in the dentinal tubules, it is difficult to eliminate this species through root canal medication.
Henceforth, the root canal sealer serves to prevent infection by acting as a barrier to further microbial challenges, to entomb any surviving bacteria in the root canal system and to stop periapical tissue fluids from reaching bacterial cells in the root canal and sustaining their survival [5]. The application of antibacterial drugs may represent a way to eradicate bacteria during root canal treatment. It has been reported that a mixture of antibacterial drugs, i.e. ciprofloxacin, metronidazole and minocycline can sterilize root dentine [6, 7].
Therefore the addition of triple antibiotic mixture to the root canal sealers may enhance the antimicrobial effect of sealers which in turn improve the success of the endodontic treatment.
the aim of this study was to evaluate the antimicrobial effect of different root canal sealers mixed with triple antibiotic mixture (TAM) against E. faecalis in experimentally infected root dentin.
Materials and Methods
This in-vitro study was carried out in the Department of Conservative Dentistry and Endodontics, and Department of Microbology, Mamata Dental College, Khammam, Telanga State, India. Ethical clearance was obtained from the Research and Ethics Committee of the Mamata Dental College. Sixty extracted single rooted premolars were taken and stored in 0.01%NaOCl (Vishal Dento Care Private Limited, Ahmadabad, India.) after cleaning the root surface. Root segments with a length of 7mm were prepared by cutting off the root tip and the crown 2-3mm below the cemento-enamel junction, using rotating water-cooled diamond saw to prevent desiccation of tooth while sectioning. Each root canal was enlarged to size 2 Peeso Reamer (Dentsply) under irrigation with distilled water, resulting in the preparation of an apical box. Organic and inorganic debris including smear layer was removed by ultrasonic irrigation with 17% EDTA, (Ammdent, Mohali, India) 3% NaOCl (Vishal Dento Care Private Limited, Ahmadabad, India.) followed by 0.9% sterile saline for 4min each for complete debridement of root canal space. The specimens were sterilized by autoclaving for 20 min at 1210C (Ideal medical systems, Model AC 700 Automatic, Bangalore, India.). The specimens were then blotted dry and external root surfaces were coated with nail varnish under sterile conditions. Sterility was checked by incubating same specimens in BHI (Brain Heart Infusion) broth (Himedia laboratories, Mumbai) for 24 hrs at 370C.
Infection of root specimens: A strain of E.faecalis ATCC 29212 was used as a test organism. The root specimens were filled with 2ml of a BHI broth and inoculated with 200μl of a 24 hour-old E.faecalis suspension. Under strict asepsis, the bacterial suspension was changed every second day for a period of three weeks.
After incubation the first microbiological sampling was performed, by flooding the canal with sterile saline followed by placing a sterile 50 size H file (Dentsply) into the canal to scrape the dentin during the process. A sterile absorbent paper point was placed into the canal for 60sec and transferred into test tubes containing 1.0 ml of stable physiological saline and was shaken vigorously for 60 seconds. A serial 10 fold dilution was prepared and 0.1ml of dilution was transferred and plated on BHI agar plates (Himedia Laboratories, Mumbai). The plates were incubated (Yorco Sales Private Limited, Delhi, India.) for 24 hrs at 37oC. Bacterial growth was detected by performing colony counts to establish the level of contamination before application of sealers.
Procedure for testing: Sixty specimens were randomly divided into six groups (n=10) based on the sealer with or without triple antibiotic mixture used. The groups were as follows:
Group 1- Zinc oxide eugenol sealer (Deepak enterprises, Mumbai, India).
Group 2- Zinc oxide eugenol sealer + Triple Antibiotic Mixture (TAM).
Group 3- Apexit Plus sealer (Ivoclor, vivadent, India).
Group 4 - Apexit Plus sealer + Triple Antibiotic Mixture (TAM).
Group 5- AH Plus sealer (Dentsply India Pvt., Ltd., New Delhi, India).
Group 6- AH Plus sealer + Triple Antibiotic Mixture (TAM).
Triple antibiotic mixture was prepared by grinding three antibiotics i.e., 200 mg of doxycycline (Uniqpharma, India) 200mg of metronidazole (Ranbaxy, India) and 250mg of ciprofloxacin (Ranbaxy, India) with mortar and pestle into a fine powder. The sealers were weighed and mixed with crushed triple antibiotic mixture at 10% of sealer’s total weight. The amount of triple antibiotic mixture was based on the results of Hoelscher et al., [5]. The canal space was dried with sterile paper point and coated with sealer. A gutta-percha cone of size 90 coated with freshly mixed sealer was introduced into the prepared canal with tweezers until fully seated. All specimens were incubated at 37oC for seven days.
Microbiological analysis: On completion of incubation obturating material was removed from all specimens with sterile Peeso Reamer size 2. A new sterile Peeso Reamer size 5 (ISO Size 150) was then used to collect dentin powder (300μm into the dentine) from each canal.
The dentin powder was obtained from each specimen, in the following manner.
Each specimen was held in sterile forceps and Peeso Reamer was introduced into the canal in an, in & out motion. Hence the cut dentinal powder was collected in the sterile petridish which was placed below the specimen. Using a sterile, plastic universal micropipette, 1ml of BHI broth was added to each petridish to assist the collection of the dentin powder. The BHI broth with the dentin powder was aspirated with a universal pipette and carried to a small, sterile test tube and vortexed for 10 seconds. Thus, the whole experiment gave a total of 60 test tubes that were then plated onto 60 separate BHI agar plates by using standard loupe method. The agar plates were incubated at 37°C for 24 h. Following incubation, visible colonies were counted and the total Colony Forming Units (CFU) were calculated by using colony counter (Yorco Sales Private Limited, Delhi, India.). Initial and final colony counts of different groups were shown in [Table/Fig-1,2,3,4,5 and 6].
Initial and final colony counts of group 1.
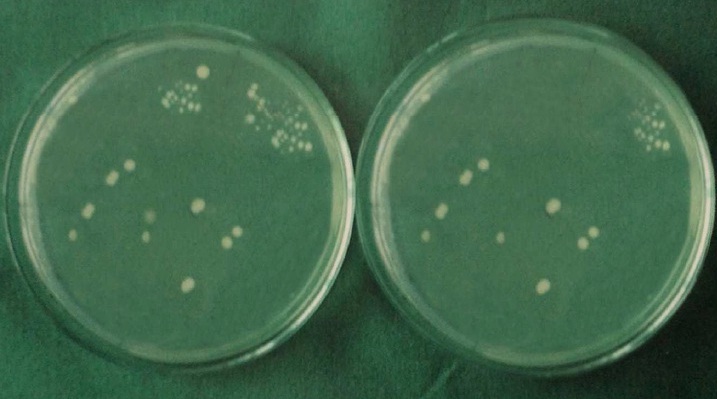
Initial and final colony counts of group 2.
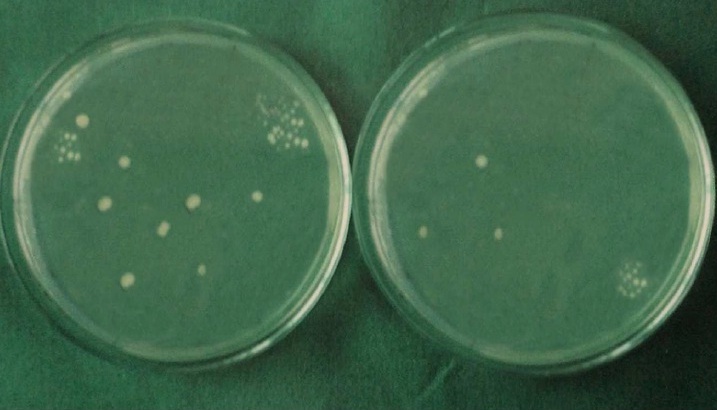
Initial and final colony counts of group 3.
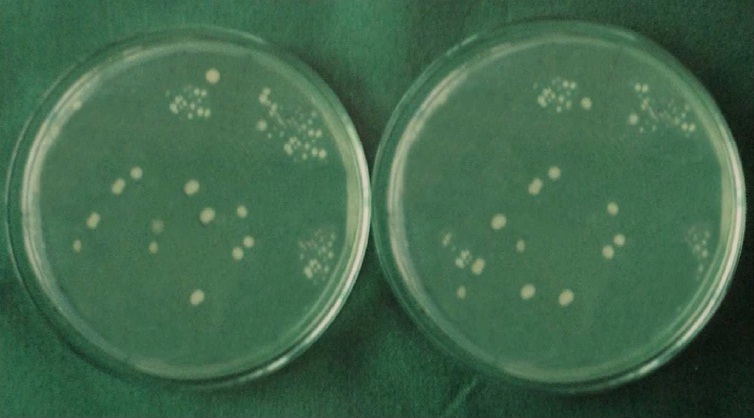
Initial and final colony counts of group 4.
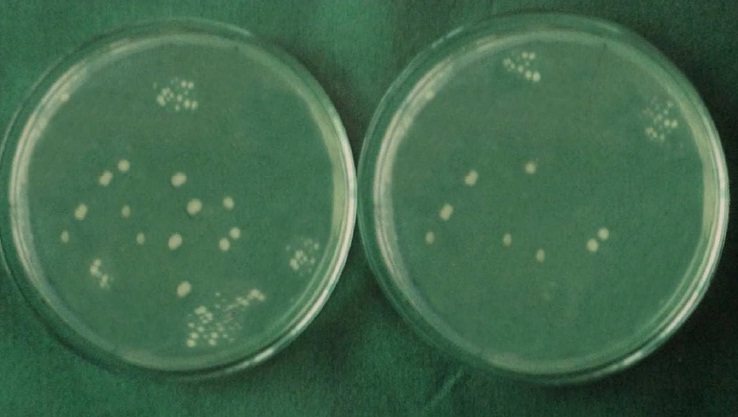
Initial and final colony counts of group 5.
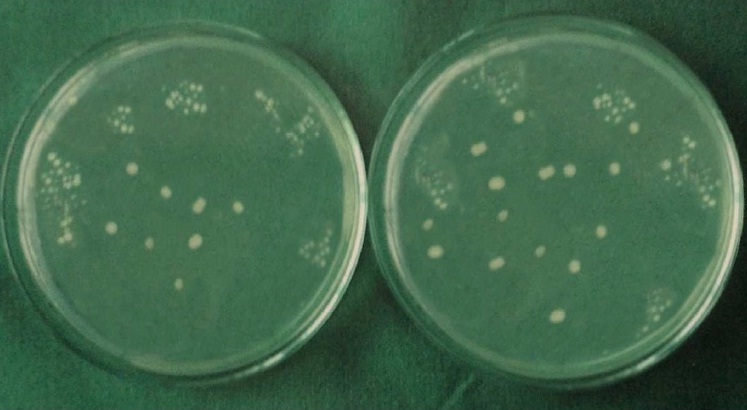
Initial and final colony counts of group 6.
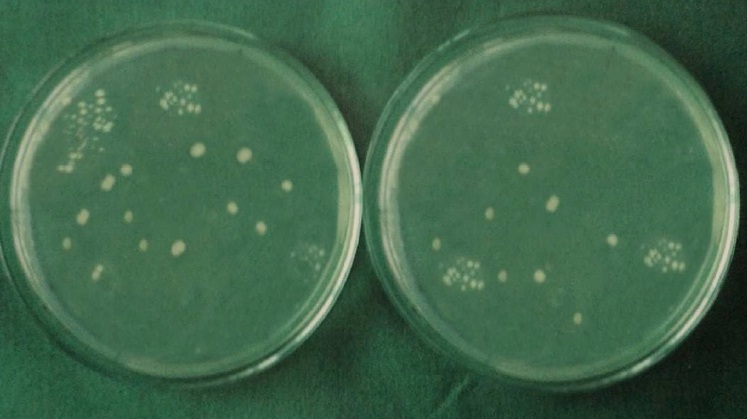
The following calculation was used:
The diameter of the loupe was 0.004 mm which holds 1/250 ml of bacterial suspension.
N - Organisms in 1/250 ml
N- The number of colonies, which is equivalent to the number of microorganisms, because one microorganism forms one colony.
X- Number of colony forming units calculated = N X 250
The antimicrobial effect was measured by comparing the Percentage Reduction in Colony Counts (% RCC) before and after sealer application.
The percentage reduction in colony counts was calculated by using the following formula:
Percentage Reduction in Colony Count= (Initial count – Final count)/Initial count X 100.
Statistical Analysis
One-way analysis of variance and tukey’s post hoc multiple comparisons were done using software Graph Pad Prism 3.
Results
[Table/Fig-7,8] shows the mean %RCC and multiple comparisons of all groups. In the present study the level of significance was set at p=0.05. The antimicrobial effect of sealers was improved when TAM was added to each individual sealer. ZnOE + TAM group showed maximum antibacterial effect (mean % RCC 97.890) and AH Plus sealer showed least antimicrobial effect (mean %RCC 57.020).
Showing the mean values and standard deviation of %RCC (One way ANOVA).
| Group | Mean | SD | p-value |
|---|
| 1 | 90.980 | 5.9632 | 0.001* |
| 2 | 97.890 | 1.8448 |
| 3 | 78.470 | 11.8744 |
| 4 | 83.870 | 5.8544 |
| 5 | 57.020 | 7.3767 |
| 6 | 74.990 | 8.3152 |
*p<0.05 indicates significant difference between groups and within the groups
Multiple comparisons among different groups by Tukey’s post hoc test.
| Group | Comparison with other groups | P Value |
|---|
| 1 | 2 | 0.324 |
| 3 | 0.006 |
| 4 | 0.293 |
| 5 | 0.001 |
| 6 | 0.001 |
| 2 | 1 | 0.324 |
| 3 | 0.001 |
| 4 | 0.001 |
| 5 | 0.001 |
| 6 | 0.001 |
| 3 | 1 | 0.006 |
| 2 | 0.001 |
| 4 | 0.596 |
| 5 | 0.001 |
| 6 | 0.903 |
| 4 | 1 | 0.293 |
| 2 | 0.001 |
| 3 | 0.596 |
| 5 | 0.001 |
| 6 | 0.109 |
| 5 | 1 | 0.001 |
| 2 | 0.001 |
| 3 | 0.001 |
| 4 | 0.001 |
| 6 | 0.001 |
| 6 | 1 | 0.001 |
| 2 | 0.001 |
| 3 | 0.903 |
| 4 | 0.104 |
| 5 | 0.001 |
*p<0.05 indicates significant difference between groups and within the groups
Discussion
Endodontic treatment outcome depends on successful reduction of the associated microorganisms and also an effective seal to prevent further recontamination [6]. E.faecalis was chosen as the test species because of its possible microbial role in persistent root canal infection [7]. Human permanent single rooted premolars were used, to simulate the clinical scenario. The experimental method used in this study is a modification of the experimental design described by Haapasalo and Orstavik et al., [8], who found that E.faecalis is suitable for experimental penetration into dentinal tubules, and three weeks of incubation with E.faecalis has been shown to produce a dense infection of the dentinal tubules, easily reaching 300μm-400μm [9]. Therefore a dental shaving was collected from 1mm (1000μm) inner surface of root canal. An ideal endodontic sealer should be biocompatible, dimensionally stable and have a strong, long lasting antimicrobial effect [10–12]. But some commercially available sealers do not have the antimicrobial effect. Adding antibiotics to a sealer can enhance their antimicrobial effect and could provide an important advantage in reducing the critical concentration of microbes necessary for a favorable host response [13].
Because of the complexity of the root canal infection it is unlikely that any single antibiotic could result in effective sterilization of the canal. More likely a combination would be needed to address the diverse flora encountered. A combination of antibiotics would also decrease the likelihood of the development of resistant bacterial strains. A mixture of ciprofloxacin, metronidazole, and minocycline has been shown to be very effective in eliminating endodontic pathogens [14,15]. Sato et al., and Hoshino et al., reported that the antibacterial efficacy of the drugs individually is not significant, but in combination, these drugs were able to inconsistently sterilize all samples [16,17]. Therefore triple antibiotic mixture which is combination of ciprofloxacin, doxycycline and metronidazole was used. Because of the discoloration effect of minocycline it was replaced by doxycycline [18].
In the present study zinc oxide eugenol sealer exerted stronger antimicrobial effect against E. faecalis, as it consist of eugenol in the formulation which is a potent antimicrobial agent [19].
Zinc oxide eugenol+TAM inhibited most of the bacterial growth. Addition of TAM to the zinc oxide eugenol sealer increased its antimicrobial effect. An antibiotic enhanced sealer that disrupts the microbe’s environment rather than merely entombing them and maintains bactericidal properties beyond its setting time could be monumental in the outcome of initial endodontic therapy and in preventing reinfection [20].
Apexit Plus is a calcium hydroxide based sealer, exhibited good antimicrobial effect but it is less than that of ZnOE sealer. The antimicrobial effect of this sealer is due to the release of hydroxide ions, which raise the pH to above 12.5. As the calcium hydroxide sealer sets, the pH declines to about 9.14, resulting in loss of the sealer’s effectiveness. The limited antibacterial activity of calcium hydroxide sealer might be attributed to a lack of sufficient pH elevation, limited solubility, and infusibility of calcium hydroxide into dentinal tubules and possibly buffering ions present in the tubules [21,22].
Apexit Plus sealer mixed with TAM showed better antimicrobial effect than Apexit Plus sealer alone, addition of antimicrobial agents increased the antimicrobial effect of sealer. AH plus sealer is a resin based sealer. The antimicrobial effect of AH plus sealer was least than rest of the groups. AH plus showed some antibacterial effect against E. faecalis. The low antimicrobial effect of AH plus against E. faecalis might be ascribed to the minimal amount of formaldehyde released over time [22]. AH plus sealer + TAM showed better antimicrobial affect than AH Plus sealer alone, but it is even less than that of other sealers tested.
Endodontic sealers should have maximum antimicrobial effect to completely eradicate the microorganisms left in the root canal space even after biomechanical preparation. Hence antimicrobial agents like TAM were added to enhance the antimicrobial effect of sealer which will improve the endodontic treatment outcome.
Limitation
There are some limitations in the study like when the triple antibiotic mixture was added to sealer the setting time, working time, flow rate, solubility, film thickness and dimensional stability after setting, might be get altered which are important for the sealer to penetrate into dentinal tubules. Hence further studies are required to study the physical properties of the sealer in combination with triple antibiotic mixture and amount of antibiotic release. Harikumar et al., reported that no statistical difference in the push out bond strength of sealer with or without antibiotics [23].
Conclusion
Within the limitations of the study it was concluded that all the sealers tested in the study showed increased antimicrobial effect against E.faecalis, when they are mixed with triple antibiotic mixture. In the clinical scenario inspite of thorough chemo mechanical preparation of root canal system, persisting bacteria may cause endodontic reinfections, therefore the endodontic sealer with antibiotics are important for complete eradication of bacteria. And as the endodontic infections are polymicrobial in nature, it is of utmost importance to use antimicrobial agents with endodontic sealer which may reduce the chances of endodontic failures.
*p<0.05 indicates significant difference between groups and within the groups
*p<0.05 indicates significant difference between groups and within the groups