The increase in the incidence of cryptococcosis in India is a serious threat and it is estimated that, this increase in incidence of cryptococcal meningitis is 0.15% in normal hosts, whereas it is as high as 6.5% in patients with AIDS [1,2]. Two varieties of C. neoformans, namely neoformans and gatti are common and neoformans being found in pigeon droppings and causes disease in immunocompromised patients including AIDS patients. Infection is acquired by inhalation of basidiospores, and is usually self-limiting and asymptomatic in an immunocompetent host [3]. More than hundred years after its discovery, because of the AIDS, cryptococcosis is causing more suffering now than at any time in history [4]. The incidence among patients with AIDS has been estimated to be 6 to 10% in developed countries [5]. Cryptococcal meningitis has been reported as the most common opportunistic infection in patients with HIV/AIDS in India and accounts for 2-7% of all opportunistic infections in large cohorts like Mumbai (4-7%), Chennai (2%) and Delhi (3.7%) [6,7]. Sporadic reports of Cryptococcal meningitis in apparently healthy host were reported from different parts of India [8]. Majority of isolates from HIV positive patients from India are serotype A; C. neoformans var. grubii being consistent with the serotype prevalent worldwide [9]. Infection can be diagnosed in the laboratory by direct demonstration of capsulated yeast in the CSF of patient by negative staining methods like India Ink or Nigrosin but it is quite insensitive technique, since it is based on the operator’s skill. Although, microscopy and culture are highly specific, these suffer low sensitivity of 50–80% [10]. Culture requires expertise; large volume of samples and it is time taking also. The latex agglutination test is a more sensitive method but may still yield false-positive and negative results [11,12]. Application of DNA probes and PCR techniques to diagnose and identify isolates of cryptococcus strains has also been described in different forms of cryptococcosis [4,10,13–15]. The latex agglutination test is rapid, and provides results within minutes. The sensitivity is quite high but suffers a drawback like false positive and negative results [16–18]. Most false positive results are caused by the presence of rheumatoid factor, which is eliminated by treating the specimen with pronase or dithiothreitol or boiling it with EDTA [19]. The LAT suffers additional problem of subjective errors and often results and grading may vary at individual levels espcially in case with borderline results. Enzyme Immunoassay (EIA) can also be used as alternate method to detect C. neoformans capsular polysaccharide antigen in patient’s samples. There are studies, which suggest low cross reactivity of EIA [20].
Polymerase Chain Reaction (PCR) techniques have greatly improved the diagnosis of cryptococcal meningitis. But the amplification techniques can present problems of sensitivity and specificity [21]. But many modifications and improvements in this technique have demonstrated that nested PCR is a sensitive, specific and that it represents a promising method to be used in the analysis of CSF samples from patients suspected of having cryptococcal meningitis [14]. It is an excellent alternative diagnostic modality for early and prompt diagnosis of cryptococcal meningitis as compared to conventional methods and not only it is rapid, but can detect low level of fungal load in small volume of samples [22–24].
Materials and Methods
Patients
A total of 200 cases of all age groups, including 14 HIV positive and 186 negative, and clinically suspected of having meningitis, were included in the study between November 2009 and September 2011 at Department of Microbiology, SGPGIMS Lucknow, Uttar Pradesh India. It is a hospital-based study and we have considered sample size consisting of all the patients who were admitted in the duration of the study in this hospital fulfilling the inclusion criteria. Patients with fever, headache, vomiting, altered sensorium and with signs of meningeal irritation were included in the study, while cases of meningitis like bacterial, viral and of unknown etiologies were excluded from the study. 4.3% of the cases were between age group 0-20, 65.2% between 21-50, and 30.3% between 51-80 age group.
Lat
3-5 ml of CSF samples were collected by lumbar puncture under strict aseptic precautions. Samples were aliquoted in two parts, one for antigen detection, culture and negative staining, and other for PCR, and stored at -80°C until subjected to molecular testing. CSF of one aliquot was centrifuged at 1500 g for 10 minutes and supernatant transferred to another aliquot for antigen testing and deposits for culture and India ink. The test was performed in titers upto as low as 1:8. Antigen testing was done using the CALAS Test Kit (Cryptococcal Antigen Latex Agglutination System, Biomerieux, Meridian Bioscience, Inc., Cincinnati, Ohio). The test utilizes latex particles coated with anti-cryptococcal globulin (Detection Latex). The detection latex reacts with the cryptococcal polysaccharide antigen causing a visible agglutination. Latex particles coated with normal globulin (Control Latex) act as one of the control reagents. Non-specific interference was detected by the Control Latex reagent and removed by pronase [25].
India ink, Culture and Biochemical identification
LAT positive samples were subjected to direct microscopy in India ink, and culture on Sabouraud Dextrose Agar (SDA). L-Canavanine Glycine Bromothymol Blue Agar (CGB Agar) was used for differentiation between C. neoformans var. neoformans and var. gattii. Caffeic acid agar was also used to identify cryptococcus neoformans which produces brown coloured colonies. Biochemical identification was done using urease test, nitrate assimilation test and sugar assimilation test using galactose, maltose, sucrose, trehalose, d-xylose, melebiose, raffinose, cellobiose, inositol, d-arabinose, and d-mannitol.
Template DNA preparation
A 500 μl of the CSF sample was taken in a sterile micro centrifuge tube and centrifuged at 5000 rpm for 10 minutes. After centrifugation the supernatant was transferred into another sterile micro centrifuge tube. The deposit with 200 μl of CSF was sonicated in a sonicator (Elmasonic S40H; 37 KHz Ultrasonic High performance transducer). The instrument was filled with distilled water up to the mark and the instrument was degassed for 30 minutes. After degassing, the micro centrifuge tube-containing sample (200 μl) was kept in a tube stand placed inside the sonicator. The sample was sonicated for 30 minutes and then sample was removed from water tank. The deposit containing cryptococcal DNA was used for the polymerase chain reaction.
Primers and PCR
The nested PCR was performed as per the protocol described by Rappeli P et al., 1998, with some modifications [14]. The two nested-primers pairs specific for internal transcribed spacer regions of ribosomal DNA of C. neoformans were sequentially used. In the first amplification, the primers ITS-1 (5’-CGTAGGTGAACCTGCGG-3’) and CN-4 (3’-ATCACCTTCCCACTAACACATT-5’), which resulted in an amplicon of 415bp and the second set of primers which was used after the first round of amplification was CN-5 (5’-GAAGGGCATGCCTGTTTGAGAG-3’) and CN-6 (3’-TTTAAGGCGAGCCGACGTCCTT-5’) which produced an amplicon of 116bp. The clinical isolates of C. neoformans and ATCC Candida albicans 90028 were used to standardize the nested PCR protocol. The standardized PCR technique was then validated in 200 cerebrospinal fluids.
Amplification was done with each sample reaction mixture of volume 50 μl for the assay in a PCR tube (6.5 μl of the PCR Master Mix (Taq DNA polymerase, KCl, (NH4)2SO4, MgCl2, dNTPs, ROX passive reference dye; Fermentas Life Sciences); 31.5 μl of nuclease free water (Fermentas Life Sciences); 1 μl each of the forward primer CN4 and reverse primer ITS1 (Eurofins Genomics, Bangalore India); 10μl of sonicated DNA template. The contents were mixed thoroughly and this reaction mixture was then put in PCR thermocycler (Gene Amp PCR system 9700, AB Applied Biosystems). An identical mixture was made for nested PCR except for 1 μl each of the forward primer CN5 and reverse primer CN6 (Eurofins Genomics, Bangalore India) and 1μl of DNA template from the 1st PCR amplification. After initial denaturation at 94° C for 2 min and 15 s, this 35 cycle profile consisted of 94° C for 2 min and 15 s, 50° C for 30 s and 72° C for 45 s. Final elongation was at 72°C. The PCR products were subjected to electrophoretic analysis on 2% agarose gel stained with ethidium bromide, and visualized on a UV transilluminator [26].
Results
Of total 200 cases included in the study, 46 cases reported positive by LAT, which included 14 HIV positive cases also [Table/Fig-1]. Amongst the HIV negative but LAT positive cases, included some or other kind of immune incompetencies like diabetes, systemic lupus erythematosus, carcinoma, sarcoidosis, and allograft recipients also. 2 (4.3%) of 46 LAT positive cases were in the age group of 0-20 years, 30 (65.2%) in 21-50 years and 14 (30.4%) between age group of (51-80). Of the 46 LAT positive cases, India ink, culture and nested PCR was positive in 33, 38 and 40 cases respectively. [Table/Fig-2] shows comparative distribution of test results of LAT, India ink, culture and nested PCR.
Cases positive by LAT (CALAS Test) and their HIV status.
| Total Cases(n=200) | Antigen +ve(n=46) | Antigen –ve(n=154) | HIV +ve(n=200) | HIV –ve(n=186) |
|---|
| 200 | 46 | 154 | 14 | 186 |
Comparative distributions of, antigen, India ink, Culture and PCR.
| Antigen (n=200) | India ink (n=46) | Culture (n=46) | PCR (n=46) |
|---|
| +ve | –ve | +ve | –ve | +ve | -ve | +ve | -ve |
|---|
| 46 | 154 | 33 | 13 | 38 | 08 | 40 | 06 |
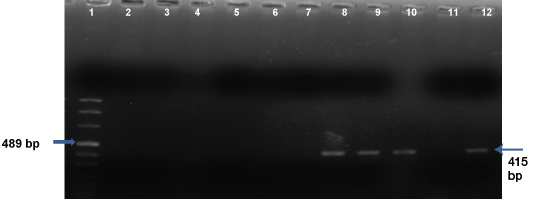
[Table/Fig-3] shows the results of the first amplification of the Nested PCR. In the first PCR amplification, the amplicon is visualized at 415bp. Lane 1 is the DNA Marker VIII, lane 12 is the positive control (Cryptococcus neoformans), lane 11 is the negative control (ATCC Candida albicans 90028), lanes 2, 3, 4, 5, 6, and 7 were negative for the PCR in the CSF samples and lanes 8, 9, and 10 showed positive amplification in the CSF samples. [Table/Fig-4] shows the results of the second nested PCR amplification where the amplicon is seen at 116bp. Lane 1 is the DNA Marker VIII, lane 11 is the positive control, lane 12 is the negative control, lanes 2, 3, 4, 5, 6, and 7, CSF specimens showing no amplicons and lanes 8, 9, and 10, the CSF samples showing the positive amplicons.
1St PCR amplification.
Lane 1DNA Marker VIII, lane 12 Positive control - C. neoformans, lane 11 Negative control - ATCC Candida albicans 90028, lanes 2, 3, 4, 5, 6, 7 Negative CSF samples, lanes 8, 9, 10 Positive CSF samples (415bp).
2nd PCR amplification (nested PCR).
Lane 1 DNA Marker VIII, lane 11 Positive control - C. neoformans, lane 12 Negative control - ATCC Candida albicans 90028, lanes 2, 3, 4, 5, 6, 7 Negative CSF samples, lanes 8, 9, 10 Positive CSF samples (116bp).

Discussion
Although there is decline in cases of cryptococcal meningitis in AIDS patients but it still represents a determinant of poor prognosis in HIV infected patients [27]. Additionally it accounts for significant morbidity in the form of severe neurological complications. Hence, rapid and accurate laboratory diagnosis is critical in successful management of cryptococcal meningitis cases [28]. We have witnessed great advances in diagnostic techniques in recent years to help establish correct diagnosis of these cases. Well-established diagnostic methods like culture sometimes become challenging and may shows negative results, which might be because of some nonviable or fastidious yeast in the sample, hence these gaps needs to be addressed [29]. The use of PCR along with established diagnostic techniques in CSF samples of cases of cryptococcal meningitis can increase the diagnostic efficacy and nested PCR can be broadly applicable [26]. The nested PCR over the years has established itself as a reliable diagnostic technique as it is sensitive, specific and moreover, the results are reproducible and can be used for monitoring the therapy in cases of different forms of cryptococcosis [14,23]. The other advantage of nested PCR is that, duration of its positivity is almost same as that of serological assays like enzyme immunoassays and LAT and this duration is longest for CSF followed by serum and urine, but nested PCR is preferred over serology because of its highest specificity [22].
The results of culture were almost concordant with that of PCR and out of 46 LAT positive samples culture and PCR was positive in 38 and 40 samples respectively. The number of cells/ml required for culture, LAT and PCR is 100, 50 and 10 respectively and in our study the culture was negative in 2 PCR positive samples, which might be because of absence of adequate number of cells required for the culture to come positive [22]. Latex agglutination although more sensitive, yields false positive and negative results but it is rapid and help establish the diagnosis in cases with negative microscopy and culture. The six LAT positive samples, which the nested PCR failed to detect, might be due to the presence of low titer of antigen rather than live yeast cells, or false positive results. Among 38 culture positive samples, biotyping on CGB agar revealed 36 isolates as C. neoformans var. neoformans and 2 isolates as var. gattii. Compared to other tests, LAT and PCR have higher sensitivity than direct microscopic methods; hence PCR should be introduced as a regular adjunct to serological methods like LAT [22]. Microscopy and culture although 100% specific, have low sensitivity and depends on the operators skill especially in case of microscopy.
Conclusion
Thus, we conclude here that although genomic methods like PCR are expensive and confined to only higher centers of India, it should always preferably be used, wherever available, while establishing diagnosis of cases of cryptococcal meningitis and thus reducing morbidity and mortality.