Candidal Vertebral Osteomyelitis in the Midst of Renal Disorders
Anusha Gopinathan1, Anil Kumar2, Srivatsa Nagaraja Rao3, Krishna Kumar4, Shamsul Karim5
1 Clincial Assistant Professor, Department of Microbiology, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
2 Clinical Professor, Department of Microbiology, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
3 Senior resident, Department of Orthopaedics, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
4 Clinical Associate Professor, Department of Orthopaedics, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
5 Professor and Head, Department of Microbiology, Amrita Institute of Medical Sciences, Kochi, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anusha Gopinathan, Clincial Assistant Professor, Departmentof Microbiology, Amrita Institute of Medical Sciences, AIMS Ponekkara, Edap-pally, Kochi, Kerala-682041, India.
E-mail: anushag20693@aims.amrita.edu
Vertebral osteomyelitis also known as discitis/pyogenic spondylitis refers to inflammation of the vertebral disc space. It is commonly seen in men and adults more than 50 years of age. Fungal osteomyelitis is a rare scenario compared to its bacterial counterpart. Spinal epidural abscess is a dangerous complication associated with vertebral osteomyelitis. Here, we report two cases of vertebral osteomyelitis caused by Candida tropicalis in patients with renal disorders (stage 5 chronic kidney disease and nephropathy). One of the case discussed here presented with spinal epidural abscess. Both the patients were started on antifungal therapy. One patient responded to treatment while the other was lost to follow up.
Candida tropicalis, Chronic renal failure, Discitis, Epidural abscess, Fungal osteomyelitis, Spondylitis
Case Reports
Case 1
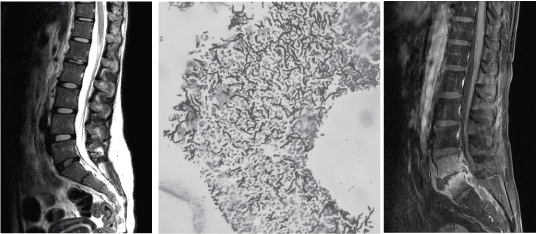
A 19-year-old female nursing college student from Kottayam complained of backache for one week duration. She was a known case of systemic hypertension and chronic kidney disease (stage 5) who was on corticosteroid treatment for one year. She was then put on haemodialysis for the past one year and has been on evaluation for renal transplantation for the last one year. On examination her vitals were stable, pallor was present. Examination of the spine revealed no local tenderness, no focal neurological deficit, straight leg test was negative, spinal movements were reduced. Laboratory investigations revealed normal leucocyte count (10,600/cubic mm), elevated erythrocyte sedimentation rate (ESR – 64mm/hr) and elevated C reactive protein (CRP – 16.17 mg/L). X ray lumbosacral spine showed reduced disc space between L1 & L2. Magnetic Resonance Imaging (MRI) lumbosacral spine T2 sagittal image showed bright signals within marrow of L1 vertebral body and mild contrast enhancement was noted in L1-L2 intervertebral disc [Table/Fig-1a]. Trans foraminal interbody fusion and biopsy of L1-L2 intervertebral disc was performed. Histopathology of the biopsy tissue revealed inflammatory granulation tissue. Gomori Methenamine Silver (GMS) stained slide showed fungal hyphae [Table/Fig-1b]. Gram staining showed presence of inflammatory cells with no bacteria and culture on Blood and MacConkey agar under aerobic conditions was negative. Candida tropicalis was isolated from the biopsy tissue when cultured onto Sabaroud’s Dextrose Agar (SDA). The yeast was isolated on day three of culture and was identified using BBLTM CHROM agar, and VITEK 2.0(Biomerieux). Antimicrobial susceptibility testing performed with VITEK 2.0 (Biomerieux) showed that the isolate was susceptibile to amphotericin (MIC <0.5), fluconazole (MIC 2), voriconazole (MIC 0.25), flucytosine and itraconazole (MIC <0.125). Blood culture was sterile. She was treated with Inj Amphotericin B (1mg/kg/day) for 14 days followed by oral fluconazole 200mg daily for three months. Follow up MRI lumbosacral spine T2 sagittal image was done after three months and showed no evidence of any abnormal collection / disc enhancement. No intervertebral disc was seen between L1-L2 [Table/Fig-1c]. The total leukocyte count was normal (4830/cubic mm), ESR 14mm/hr and CRP 1.07 mg/L. She continues to remain stable till date.
(a) Case 1: Preoperative MRI Lumbosacral spine: T2 sagittal image showing bright signals within marrow of L1 vertebral body. Mild contrast enhancement noted in L1-L2 intervertebral disc. (b) Histopathology examination of biopsy tissue by Gomori methenamine silver staining showing fungal hyphae (10X). (c) Case 1: Postoperative MRI Lumbosacral spine: T2 sagittal image showing no evidence of any abnormal collection / disc enhancement. No intervertebral disc seen between L1-L2.
Case 2
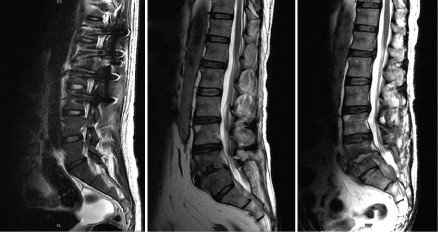
A 64-year-old male from Kollam, known case of type II diabetes with nephropathy presented with severe back pain of one month duration. He had undergone bilateral DJ stenting in the past for obstructive uropathy and is currently on haemodialysis. Patient had pain on walking and sitting. On examination, sensation was decreased to pain, touch and temperature over left L5 and S1 dermatome, motor system examination revealed decreased left hip extension (grade 4+), sluggish deep tendon reflexes and straight leg test was 600 on the left side. Tenderness was present over the dorsolumbar spine. There were no signs of meningeal irritation. Patient’s gait was normal. Laboratory investigations revealed elevated urea (37.1 mg/dl), creatinine (1.43mg/dl), CRP (17.89 mg/L), ESR (46mm/h), Hb A1C (7.5%), leukocyte count (13,700/ μl). Magnetic Resonance Imaging (MRI) lumbosacral spine Saggital T2 image showed signal intensity changes in the L5-S1 intervertebral disc with loss of disc integrity. Heterogeneous signal intensity changes were noted in L5-S1 end plate [Table/Fig-2a]. MRI Lumbosacral spine Saggital T1 image with contrast showed signal intensity changes in the L5-S1 intervertebral disc with loss of disc integrity. Heterogeneous signal intensity changes were noted in L5-S1 end plate. Contrast enhancing peridiscal epidural collection (more in the posterior aspect) - likely abscess was seen [Table/Fig-2b]. The findings were suggestive of infective spondylodiscitis with epidural abscess. Patient underwent L4-L5 discectomy with drainage of abscess and L3-S1 instrumented posterior lumbar interbody fusion was performed. The purulent material drained was sent for bacterial and fungal culture. Gram staining and culture onto blood and MacConkey agar did not yield any results. Fungal culture on Sabaroud’s dextrose agar yielded Candida tropicalis. The yeast was identified using BBLTM CHROM agar, and VITEK 2.0 (Biomerieux) and was found susceptible to amphotericin B (MIC 0.5), fluconazole (MIC <=1), voriconazole (MIC<= 0.12) and flucytosine (MIC <=1). Histopathology of the biopsied tissue from surgery showed features suggestive of granulomatous inflammation with suppuration. Blood culture was sterile. Patient underwent L4-L5 discectomy with drainage of abscess and L3-S1 instrumented posterior lumbar interbody fusion was performed. He was treated with oral fluconazole 200mg twice a day and then continued with fluconazole 150mg once daily for 6 weeks. Follow up MRI lumbosacral spine saggital T2 image performed after six months showed soft tissue component of epidural abscess measuring 2x1.9cm, and causing posterior compression of the thecal sac and cauda [Table/Fig-2c]. Patient was lost to follow up.
(a) Case 2: Preoperative MRI Lumbosacral spine: Saggital T2 image showing signal intensity changes in the L5-S1 intervertebral disc with loss of disc integrity. Heterogeneous signal intensity changes are noted in L5-S1 end plate. (b) Case 2: Preoperative MRI Lumbosacral spine: Saggital T1 image with contrast showing signal intensity changes in the L5-S1 intervertebral disc with loss of disc integrity. Heterogeneous signal intensity changes are noted in L5-S1 end plate. Contrast enhancing peridiscal epidural collection (more in the posterior aspect) - likely abscess seen. (c) Case 2: Postoperative MRI Lumbosacral spine: Saggital T2 image showing soft tissue component of epidural abscess measuring 2x1.9cm, and causing posterior compression of the thecal sac and cauda.
Discussion
The incidence of vertebral osteomyelitis/ pyogenic spondylitis is 1: 250,000 to 1:450,000 [1,2]. This condition which commonly ails the old (>50 years), can also be seen in young adults especially those with history of intravenous drug abuse [3]. Urinary tract infections and transient bacteraemia are commonly associated with this condition. It can also be caused by direct inoculation to the spine by spinal surgery and penetration wounds [4]. The source of infection is often unknown in most cases. This was the scenario in both are cases too.
Candida albicans accounts for 62% of vertebral osteomyelitis cases caused by Candida species followed by Candida tropicalis (19%), Candida glabrata (14%) and Candida parapsilosis (4%) [4]. Osteomyelitis caused by Candida species is most often seen in the vertebra (40.3%), sternum (7.8%), costochondral (2.6%), finger phalanx (2.6%) and mandibule (2.6%) [5]. Candida which is considered as a commensal is known to cause infections in the presence of risk factors such as long term antibiotic therapy, presence of intravenous catheters, parenteral nutrition, haemodialysis, surgery, burns, organ transplantation, HIV infection, corticosteroid therapy, myeloperoxidase deficiency and neutropenia [5,6]. Both our patients had chronic renal disorders and were on haemodialysis before the onset of vertebral osteomyelitis.
The pathogenesis of vertebral osteomyelitis is mostly due to hematogenous seeding and less likely from contiguous focus of infection. In both our cases, blood culture was sterile and there was no other focus of infection in the patients. The presentation of Candidal vertebral osteomyelitis is most often as pain in the lumbar spine for a duration of more than one month, with fever seen in one third of patients and limitation of motion with or without neurological deficits [6,7]. Neurological deficits are commonly seen in patients where the infection has spread to the epidural space [8]. The laboratory findings of these patients are often non-specific, with normal WBC count, elevated ESR and elevated CRP. All the above findings were seen in both our patients. Epidural abscess secondary to vertebral osteomyelitis similar to our case was also seen in reports from Turkey and Korea. Candida albicans and Candida parapsilosis were the etiological agents respectively in these patients [4,9]. Diabetes mellitus, chronic renal failure, intravenous drug abuse, alcoholism and cancer are conditions associated with spinal epidural abscess [10]. Our patient who was diagnosed with spinal epidural abscess had type II diabetes and was on haemodialysis for chronic renal failure.
Imaging modalities such as MRI(Magnetic Resonance Imaging), CT(Computed Tomography) scan and radionuclide studies are useful for diagnosis [6]. In both our patients MRI with contrast was used to clinch the diagnosis. Culture of bone biopsy or pus is required for microbiological diagnosis. Needle biopsy or open biopsy is performed to obtain the specimen with open biopsy being the preferred diagnostic modality. In both our cases Candida tropicalis was isolated on culture of the specimen. Identification of the fungal pathogen to the species level is required to determine the presence of intrinsic resistance to antifungal therapy [5].
Treatment options for patients with vertebral osteomyelitis are parenteral amphotericin for 4 – 6 weeks followed by prolonged course (2-6 months) of oral azole therapy [11]. Ketoconazole can also be used as therapy in patients with history of drug abuse and disseminated candidiasis syndrome. Surgery may be required in most patients. Treatment is discontinued when the patient shows clinical response with normalization of ESR and MRI imaging. Among our cases, the first patient was treated with parenteral amphotericin for 2 weeks followed by oral fluconazole for 3 months, while the second patient was treated with fluconazole for 12 weeks. Fusion of vertebrae was performed in both the patients. Prognosis of patients with vertebral osteomyelitis is good. The cure rate for Candidal vertebral osteomyelitis is 85% [6]. Patients with epidural abscess who do not have paralysis or its presence less than 36 hours are known to have good prognosis [8]. This was the scenario in our patient too.
Conclusion
Vertebral osteomyelitis is a rare clinical condition which on delay in diagnosis can lead to deadly complications such as epidural abscess. This study shows the importance of evaluating patients with vertebral osteomyelitis for fungal pathogens. Though it is often difficult to obtain proof, hematogenous origin is considered as the common source of infection in these cases. Surgery and appropriate antifungal therapy play a major role in treatment of these patients.
[1]. Digby JM, Kersley JB, Pyogenic non-tuberculous spinal infection: an analysis of thirty casesJ Bone Joint Surg Br 1979 61(1):47 [Google Scholar]
[2]. Beronius M, Bergman B, Andersson R, Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990-95Scand J Infect Dis 2001 33(7):527 [Google Scholar]
[3]. Sapico FL, Montgomerie JZ, Pyogenic vertebral osteomyelitis: report of nine cases and review of the literatureRev Infect Dis 1979 1(5):754 [Google Scholar]
[4]. Cho K, Lee SH, Kim ES, Eoh W, Candida parapsilosis spondylodiscitis after lumbar discectomyJ Korean Neurosurg Soc 2010 47(4):295-97. [Google Scholar]
[5]. Arias F, Mata-Essayag S, Landaeta ME, Capriles CH, Pérez C, Núñez MJ, Candida albicans osteomyelitis: case report and literature reviewInt J Infect Dis 2004 8(5):307-14. [Google Scholar]
[6]. Miller DJ, Mejicano GC, Vertebral osteomyelitis due to Candida species: case report and literature reviewClin Infect Dis 2001 33:523-30. [Google Scholar]
[7]. Darouiche RO, Spinal epidural abscessN Engl J Med 2006 355(19):2012-20. [Google Scholar]
[8]. Grewal S, Hocking G, Wildsmith JAW, Epidural abscessesBr J Anaesth 2006 96(3):292-302. [Google Scholar]
[9]. Ozdemir N, Celik L, Oguzoglu S, Yildirim L, Bezircioglu H, Cervical vertebral osteomyelitis and epidural abscess caused by Candida albicans in a patient with chronic renal failureTurk Neurosurg 2008 18(2):207-10. [Google Scholar]
[10]. Mackenzie AR, Laing RB, Smith CC, Kaar GF, Smith FW, Spinal epidural abscess: the importance of early diagnosis and treatmentJ Neurol Neurosurg Psychiatr 1998 65:209-12. [Google Scholar]
[11]. Ramos A, Huddleston PM, Patel R, Vetter E, Berbari EF, Vertebral osteomyelitis due to Candida species: a cohort study and review of the literatureOpen J Orthop 2013 3:81-9. [Google Scholar]