Biphasic Effect of Rifampicin on Bilirubin- A Case Report
Manigandan Gopi1, Mandalam Subramanian Seshadri2
1 Consultant Physician, Department of Internal Medicine, Thirumalai Mission Hospital, Ranipet, Tamilnadu, India.
2 Consultant Physician & Endocrinologist, Department of Internal Medicine, Honorary Medeical Director, Thirumalai Mission Hospital, Ranipet, Tamilnadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. G. Manigandan, 33 B thesai Street, Walajapet-632513, Vellore District, Tamilnadu, India.
E-mail: lyfdiver@gmail.com
Drug induced hepatitis is a major problem which a physician encounters in his clinical practice. In view of increasing incidence of tuberculosis in our country a large number of infected individuals are started on Antituberculous (ATT) drugs and rifampicin is invariably part of the regimen. One of the major adverse effects of ATT drugs is drug- induced hepatitis which is characterized by elevation of liver enzymes and bilirubin. Hepatotoxicity is usually idiosyncratic or dose-dependent. Rifampicin causes transient elevation of transaminases in 10-20 percent of individuals and this does not warrant dose adjustments of the drug. Rarely rifampicin can lead to severe hepatitis with hyperbilirubinaemia and marked elevations of SGOT and SGPT and in some patients this can be fatal. The exact mechanism of Rifampicin induced hepatotoxicity is not known but it is postulated to be due to idiosyncratic reaction to rifampicin metabolites which may be directly toxic or induce an immunologically mediated liver injury. Rarely rifampicin may cause hyperbilirubinaemia without enzyme elevation. Here we report a patient with bilateral pulmonary tuberculosis who developed transient severe indirect hyperbilirubinaemia on rifampicin. On review of relevant literature we find that rifampicin can have a biphasic effect on bilirubin, an initial increase in indirect bilirubin and later normalization of bilirubin. We have reported this case because of its rarity in clinical practice.
Antitubercular drugs, Drug induced hepatitis, Hyperbilirubinaemia
Case Report
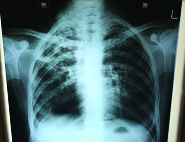
A 24-year-old female working in the garment industry presented with low grade fever for the past four months, cough and 10 kg weight loss in four months. Her Chest X-ray showed bilateral pulmonary TB [Table/Fig-1] and sputum AFB smear was repeatedly positive. She had been started on Category 1 anti-Tβ regimen under DOTS as per RNTCP guidelines. After three days of starting the medication she noticed yellowish discoloration of sclera but in spite of this she continued the drug for a further two weeks. Subsequently she had nausea and vomiting and so came to our hospital.
Chest X ray showing Active Pulmonary tuberculosis with a cavity in right upper lobe.
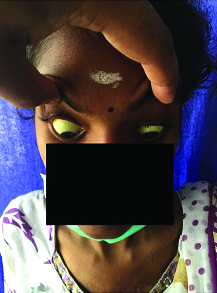
On clinical examination she was thin built, malnourished, pale and deeply icteric [Table/Fig-2]. She had a tender hepatomegaly 2 cm below the right costal margin but spleen was not palpable. She had crepitations in both infraclavicular regions. Cardiovascular system Abdomen and CNS were clinically normal. Fundus exam showed pale retina but the optic discs and vessels were normal.
Figure showing deep icterus.
Investigation:
She had anaemia with haemoglobin of 8.1 gm/dl. Peripheral smear showed microcytic hypo chromic anaemia blood picture suggestive of iron deficiency. Retic count was normal and Direct Coomb’s Test negative. LFT: Total bilirubin: 13.5, Direct bilirubin -3.5 & indirect bilirubin 10 mg/dl (Predominantly indirect hyperbilirubinaemia) Transaminases, LDH and alkaline phosphatase were normal. She had history of poor sun exposure and so serum 25 (OH) vitamin D was checked and found to be low (Patient sample-17 ng/ml; normal 30-100 ng/ml). Chest X ray [Table/Fig-1] showed bilateral extensive pulmonary tuberculosis with cavitation in the right upper lobe. Sputum was repeatedly positive for acid fast bacilli. HIV, HBsAg and HCV antibody tests were negative.
She was initially started on Isoniazid, Ethambutol and Ofloxacin as per the British Thorasic society guidelines [1]. She showed gradual improvement After 1 week bilirubin had come down to 1.3 mg/dl. Rifampicin was added to the regimen and the serum bilirubin checked after 1 week was 1 mg/dl. She continued to have spikes of fever and had persistent neutrophilic leucocytosis. A repeat Chest radiograph was done and this showed patchy consolidation and cavity with air-fluid level in the right upper lobe. After sputum culture she was started on Augmentin in addition, to cover superadded bacterial infection. Sputum culture showed Staphylococcus aureus sensitive to Augmentin. An ultrasound examination was done and it showed multiple tiny echogenic foci within the liver suggestive of military tuberculosis. Pyrazinamide was added after repeated LFTs showed normal values. Patient became afebrile for one week after the course of augmentin, but again had fever spikes and Streptomycin was added to the regimen following which she became afebrile within 2 weeks.
Patient continued her drugs and came for review after two months. She was afebrile and her appetite had improved. Her sputum samples became negative for AFB. She gained about 2 kg of weight. She was advised to continue and complete the course of anti TB drugs.
Discussion
Drug induced liver injury(DIH) has replaced the viral hepatitis as the most common cause of acute liver failure [2]. Drug induced liver injury is most important side effect caused by Anti TB drugs. Drug induced liver injury is diagnosed only after excluding viral hepatitis [3]. It is characterized by rise in ALT more than two times normal and a fall in the ALT on stopping the drug. Re-challenge should cause a rise in ALT [3]. Advanced age, female sex, alcoholism, hepatitis B and C virus, N acetyl transferase phenotype, increased N-acetyltransferase (NAT) activity, underlying liver disease, reduced glutathione S-transferase activity, Human Immunodeficiency Virus (HIV) infection, extensive disease and malnutrition are considered to be the major risk factors for ATT induced hepatitis [4]. Rifampicin causing drug induced hepatits occurs earlier than that caused by isoniazid and is characterized by patchy cellular involvement with marked periportal inflammation [5]. Rifampicin causing drug induced hepatitis is considered to be a part of a systemic allergic reaction [5].
McCoel et al., found that in normal subjects, the rise in the unconjugated bilirubin during the initial phase after administration of rifampicin (in the first week) closely followed the serum concentration of rifampicin [6]. This observation suggests that the drug inhibits the clearance of the bilirubin by liver. Since only the unconjugated fraction is increased, rifampicin must be interfering in one or more of the following steps in bilirubin metabolism in the liver: 1) hepatocellular uptake; 2. intracellular protein binding; or 3) conjugation of bilirubin [6]. When rifampicin therapy was continued, in the third week and fourth week of therapy, indirect bilirubin levels normalized and this observation suggests that this decrease in unconjugated bilirubin is due to increased hepatic clearance of the bilirubin induced by rifampicin [6]. This biphasic effect of rifampicin on bilirubin is similar to what we observed in our patient. She had initial indirect hyper-bilirubinaemia which necessitated temporary discontinuation of the drug and reintroduction of the drug after 1 week did not evoke this response. It has been reported previously [7] that rifampicin can occasionally cause unconjugated hyperbilirubinaemia which is only subclinical without elevation of liver enzymes. However in our patient, rifampicin caused marked clinical jaundice with unconjugated hyperbilirubinaemia without elevation of liver enzymes and such an event is quite rare. In our patient anaemia along with elevated indirect bilirubin raised the possibility of a haemolytic process. However she did not have reticulocytosis and DCT was negative excluding haemolysis as a cause for the observed hyperbilirubinaemia.
Conclusion
In patients with tuberculosis when a rifampicin containing ant TB treatment is instituted, jaundice occurring within 1 week should suggest rifampicin toxicity. However, if only indirect hyperbilirubinaemia is found and if haemolysis is excluded the patient may be restarted on rifampicin with close monitoring of bilirubin. Since Rifampicin is the most important first line anti TB drug it is very important to restart this drug in order to have a satisfactory response to ant TB therapy.
[1]. Joint Tuberculosis Committee of the British Thoracic SocietyChemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998Thorax 1998 53:536 [Google Scholar]
[2]. Ostapowicz GM, Fontana R, Schidt F, Larson A, Davern T, Steven Han H, Results of a prospective study of acute liver failure at 17 tertiary care centers in the United StatesAnn Intern Med 2002 137:947-54. [Google Scholar]
[3]. Benichou C, Criteria for drug-induced liver disorder: report of an international consensus meetingJ Hepatol 1990 11:272-76. [Google Scholar]
[4]. Sharma SK, Balamurugan A, Saha PK, Pandey RM, Mehra NK, Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during antituberculosis treatmentAm J Respir Crit Care Med 2002 166:916-19. [Google Scholar]
[5]. Kenwright S, Levi AJ, Sites for competition in selective hepatic uptake of rifampicin, flavaspidic acid, bilirubin and bromsulphthaleinGut 1974 15:220-26. [Google Scholar]
[6]. McColl KE, Thompson GG, El Omar E, Moore MR, Park BK, Brodie MJ, Effect of rifampicin on haem and bilirubin metabolism in manBr J Clin Pharmacol 1987 23(5):553-59. [Google Scholar]
[7]. Kadka J, Malla P, Jha SS, Poudel SR, The study of drug induced hepatotoxicity in ATT patients attending in national tuberculosis center in BhaktapurSAARC J Tuberculosis, Lung Dis HIV/AIDS 2009 6:17-21. [Google Scholar]