Cirrhosis is a chronic from of liver disease which is usually defined histologically by development of regenerative nodules surrounded by fibrous bands. This in turn leads to development of intrahepatic, sinusoidal portal hypertension and end stage liver disease [1]. Ascites is a known complication of cirrhosis and portal hypertension. Usually it is sterile, but when it gets infected by bacteria then the condition is termed as Spontaneous Bacterial peritonitis (SBP). SBP is an ominous complication in patients with cirrhosis. SBP can occur in any race and both sexes equally.
Harold Conn first recognized the disorder in the 1960s. Enteric organisms have traditionally been isolated from more than 90% of infected ascites fluid in SBP, suggesting that the GI tract is the source of bacterial contamination. Patients with cirrhosis who are in a decompensated state are at the highest risk of developing SBP [2].
Majority of SBP infections have been caused by aerobic gram-negative organisms (50% of these being Escherichia coli). The remainder has been due to aerobic gram-positive organisms (19% streptococcal species). One study cites a 34.2% incidence of streptococci, ranking in second position after Enterobacteriaceae. Viridans Group Streptococci (VGS) accounted for 73.8% of these streptococcal isolates [3,4].
C-Reactive Protein (CRP) was discovered by Tillet and Francis in 1930 and they proposed the hepatic synthesis of CRP. It is an acute phase protein found in the blood stream the levels of which rise in response to inflammation [5]. High sensitivity CRP (hs- CRP) test measures low levels of CRP using Laser nephelometry. This test gives immediate results within 25 minutes and also with sensitivity down to 0.04 mg/l. It is a more sensitive inflammatory marker.
Role of hs-CRP as a marker of inflammation has been extensively studied in Coronary Artery Diseases (CAD). Apart from CAD, hs- CRP is also being studied in other conditions like chronic liver diseases, collagen vascular diseases, inflammatory arthritis. As far as liver diseases are concerned CRP and hs-CRP has been studied as an inflammatory marker in infective hepatitis, alcoholic liver diseases, cirrhosis and SBP. Studies have supported the view that CRP levels increases in decompensated cirrhosis and infections in cirrhosis [6–9]. In this study we will assess the role of ascitic fluid hs-CRP level as a prognostic marker in cases of cirrhotic ascites with SBP.
To compare the levels of ascitic fluid hs-CRP in patients with SBP and without SBP, and to assess the levels of ascitic fluid hs-CRP in patients with SBP before the initiation of antibiotic therapy and on 7th day after initiation of therapy or before discharge.
Materials and Methods
The present study was carried out in the Department of Medicine at Acharya Vinoba Bhave Rural Hospital of Jawarlal Nehru Medical College, Sawangi (Meghe), Wardha for period of 2 years from September 2013 to October 2015.
The study was initiated only after obtaining permission from the institutional ethics committee.
Study Design
Prospective case control study.
Sample size
A total of 100, (50 cases, 50 controls) were studied.
Inclusion Criteria
Study group
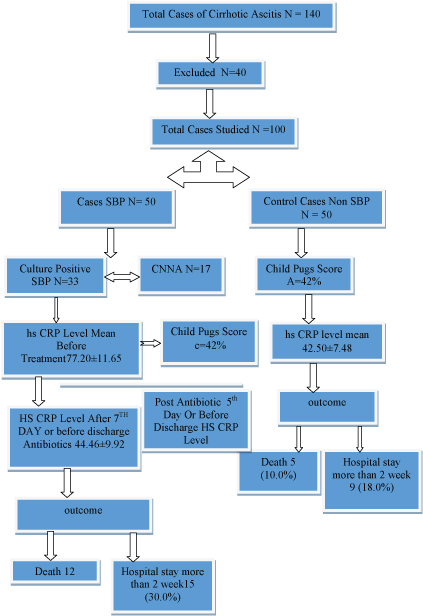
A total of 140 patients with decompensated cirrhosis admitted in medicine ward and ICU were screened for the study and out of them 40 were excluded from being taken into the study after applying the exclusion criteria given vide infra .
Exclusion Criteria
Coronary artery diseases,
Diabetes mellitus,
Collagen vascular disorders,
Any form of acute arthritis,
Acute infections,
Sepeticaemia,
Patients not giving consent.
Definition of Cases
Fifty cases of cirrhosis with ascites who satisfied the criteria of SBP. The definitive diagnosis of SBP was made if there was a positive ascitic fluid bacterial culture and an elevated ascitic fluid absolute Polymorpho Nuclear (PMN) leukocyte count of ≥250 cells/mm, in absence of any intra abdominal source of infection.
Patients with Culture Negative Neutrocytic Ascites (CNNA) with a PMN cell count ≥ 250 cells/mm3 without any intra abdominal source of infection and who were not treated with antibiotics 30 days prior to presentation were also considered as SBP. Fifty patients of decompensated cirrhosis with sterile (non SBP) ascites were taken as controls [10].
Sources of data
Patient general information (name, age, sex, address) was noted. A detailed history regarding duration of cirrhosis, amount and duration of alcohol intake, blood transfusions, intravenous drug abuse, promiscuous sexual behaviour, drug intake, investigation history in form of (serologic evaluation/USG of liver confirming cirrhosis), and prior treatment history was taken. History of fever, abdominal pain, haematemesis, malena, haematochezia, altered mentation, oliguria was noted. A detailed general physical examination along with signs of hepatocellular failure were sought and noted. Cirrhosis and Ascites was clinically diagnosed and confirmed by ultrasound of abdomen.
Laboratory investigations
Haemoglobin, TLC, Serum bilirubin, AST, ALT serum creatinine, INR, were estimated in all cases. All the patients underwent diagnostic/ therapuetic paracentesis with all aseptic precautions.
Immediately, 10 ml of ascitic fluid was inoculated into an anaerobic and aerobic blood culture bottles, bottle incubation and subsequent testing were carried out according to the hospital microbiology laboratory protocol. 10 to 20 ml of fluid was placed in a sterile container for direct microscopic examination, gram-stained film, and culture on routine laboratory media including blood, MacConkey, Mannitol salt agar plates, and thioglycollate broth. Culture-positive samples were then identified. Approximately 3 ml of fluid was placed in an EDTA tube for estimation of the total cell count and PMNL count using an automated cell counter model Sysmex Kx N 21. Ascitic fluid hs - CRP was estimated by tubidimetry method.
All patients were divided according to child pugh criteria. SBP cases were treated with standard recommended antibiotic therapy (inj cefotaxim 2 g 12 hourly for 5th days) [11]. Highly sensitive CRP levels were again estimated after 5th day of antibiotic therapy or at the time of discharge. Normal hs-CRP, ranges from 0.5 to 10 mg/l [Table/Fig-1].
Sequential flow chart of the study.
Statistical Analysis
Statistical Analysis was performed with help of Epi Info (TM) 3.5.3 which is a trademark of the Centers for Disease Control and Prevention (CDC). Test was used to test the association between different study variables under study. Test of proportion (Z-test) was used to test the significant difference between two proportions. t-test was used to test the significant difference between means. Odds ratio (OR) with 95% Confidence Interval (CI) was calculated to measure the different risk factor. Significance level was set at 0.05 (p<0.05) and confidence intervals were at 95% level.
Results
Mean age of study subjects was 46.84 ± 12.46 with no significant difference in mean age of the two groups (45.34 vs. 48.34 years). The ratio of gender was found as Male: Female: 6.1:1 (p< 0.01) [Table/Fig-2].
Baseline characteristic of cases and controls.
| Characteristics | SBP(n=50) | Without SBP(n=50) | t98- and χ2 value | p-value |
|---|
| Age | 45.34±11.73 | 48.34±12.46 | t98=1.014 | 0.32 |
| Sex | | | χ2 = 2.07 | 0.14 |
| Male | 46(92.0%) | 40 (80.0%) | | |
| Female | 4(8.0% | 10 (20.0%) | | |
| Aetiology | | | | 0.001 |
| Alcoholic | 80% | 68% | χ2 = 6.69 | |
| Other Cause | 20% | 32% | | |
| HB | 9.60±1.70 | 10.21±1.97 | 1.65 | 0.10 |
| TLC | 13.10±9.51 | 11.80±17.40 | 0.46 | 0.64 |
| Sr. bilirubin | 5.56±4.56 | 3.5±2.1 | 2.90 | 0.0046 |
| AST | 128.00±56.26 | 110.00±66.45 | 1.46 | 0.14 |
| ALT | 66.70±30.34 | 62.71±66.50 | 0.38 | 0.70 |
| SR.Creatinine | 1.8±0.70 | 1.1±0.68 | 5.07 | 0.0001 |
| INR | 2.8±1.28 | 1.93±0.60 | 4.35 | 0.0001 |
| Child Pug Score | | | χ2 = 5.12 | 0.05 |
| A | 11(22.0%) | 21(42.0%) | | |
| B | 18(36.0%) | 16(32.0%) | | |
| C | 21(42.0%) | 13(26.0%) | | |
| Ascitic Fluid HSCRP Level | 77.20±11.65 | 42.50±7.48 | t98=17.72 | 0.0001 |
| Death | 12 (24.0%) | 5 (10.0%) | Z=2.63 | P=<0.5 |
| Mean HSCRP Level Who Died | 80.20±13.65 | 44.56±8.48 | t98=15.68 | P=0.00001 |
| Hospital Stay More Than 2 Week | 15 (30.0%) | 9 (18.0%) | Z=1.99 | P =<0.05 |
| MeanHs-CRP Level in Hospital Stay More Than 2 Week | 74±11.75 | 40 ±6.42 | t98=18.56 | P =0.00001 |
Among the aetiologies of cirrhosis cases proportion of alcoholism (74%) was significantly higher (Z=9.16; p=0.001) followed by viral infection [Table/Fig-3].
Distribution of aetiology of cirrhosis.
| Aetiology | (n=100) |
|---|
| Alcoholism | 74 (74.0%) |
| HBV | 8 (8.0%) |
| HCV | 4 (4.0%) |
| Alcoholic + HBV | 7 (7.0%) |
| HCV + HBV | 2 (2.0%) |
| Cryptogenic | 10 (10.0%) |
| Autoimmune Hepatitis | 1 (1.0%) |
| Billiary Cirrhosis | 3 (3.0%) |
Proportion of Escherichia Coli (38%) was significantly higher than other organism (Z=3.50; p=0.00001). Only 4% were Enterococus SP. and 34% were CNNA [Table/Fig-4]. Corrected Chi-square test showed that there was significant association between level of hs-CRP before antibiotic therapy and groups (p=0.0001).
| Organism | No. of SBP Patient (N 50) |
|---|
| Escherichia coli | 19 (38%) |
| Klebsiella | 8 (16%) |
| Strep.Viridians | 4(8%) |
| Enterococus Sp. | 2 (4%) |
| CNNA | 17 (34%) |
The mean level of hs-CRP before antibiotic therapy (mean ± S.D.) of the patients with SBP was 77.20±11.65 mg/dl with range 56–93 mg/dl and the median was 77.50 mg/dl. The mean level of Hs-CRP before antibiotic therapy (mean ± S.D.) of the patients without spontaneous bacterial peritonitis was 42.50±7.48 mg/dl with range 22 – 58 mg/dl and the median was 42.0 mg/dl [Table/Fig-5].
Distribution of Ascitic fluid level of hs-CRP before antibiotic therapy in two groups.
| Level of Hs-CRP(mg/dl) | With spontaneous bacterial peritonitis(n=50) | Without spontaneous bacterial peritonitis(n=50) | Total |
|---|
| 20-29 | 0 | 1 | 1 |
| Row % | 0.0 | 100.0 | 100.0 |
| Col % | 0.0 | 2.0 | 1.0 |
| 30-39 | 0 | 13 | 13 |
| Row % | 0.0 | 100.0 | 100.0 |
| Col % | 0.0 | 26.0 | 13.0 |
| 40-49 | 0 | 26 | 26 |
| Row % | 0.0 | 100.0 | 100.0 |
| Col % | 0.0 | 52.0 | 26.0 |
| 50-59 | 4 | 10 | 14 |
| Row % | 28.6 | 71.4 | 100.0 |
| Col % | 8.0 | 20.0 | 14.0 |
| 60-69 | 13 | 0 | 13 |
| Row % | 100.0 | 0.0 | 100.0 |
| Col % | 26.0 | 0.0 | 13.0 |
| ≥70 | 33 | 0 | 33 |
| Row % | 100.0 | 0.0 | 100.0 |
| Col % | 66.0 | 0.0 | 33.0 |
| TOTAL | 50 | 50 | 100 |
| Row % | 50.0 | 50.0 | 100.0 |
| Col % | 100.0 | 100.0 | 100.0 |
| Mean ± s.d. | 77.20±11.65 | 42.50±7.48 | |
χ2 = 88.57; p=0.0001; S-Significant
The t-test showed that the mean level of Hs-CRP before antibiotic therapy of the patients with SBP was significantly higher than that of the patients without spontaneous bacterial peritonitis (t98=17.72;p=0.0001). The t-test showed that the mean level of Hs-CRP at 5th day or discharge after initiation of antibiotic therapy was significantly lower than that of level of hs-CRP before initiation of antibiotic therapy (t98 = 15.12;p=0.00001). Thus significant reduction in level of hs-CRP was observed [Table/Fig-6].
Comparison of level of Ascitic fluid hs-CRP before antibiotic therapy and at 5th day or discharge after initiation of antibiotic therapy of the patients with spontaneous bacterial peritonitis.
| Value of Descriptive statistics | Ascitic fluid Level of Hs-CRP before antibiotic therapy(mg/dl)(n=50) | Ascitic fluid Level of Hs-CRP at 5th day or discharge after initiation of antibiotic therapy(mg/dl)(n=50) | t98 | p-value |
|---|
| Mean ± s.d. | 77.20±11.65 | 44.46±9.92 | 15.12 | 0.00001 |
| Median | 77.5 | 42.0 |
| Range | 56.0-93.0 | 30.0-64.0 |
The mean ascitic fluid hs-CRP level in the patient, who died (80.20±13.65) and in the patient who had prolong hospital stay more than two weeks (74±11.75) was also significantly higher than the mean ascetic fluid hs-CRP level of the controls who died (44.56±8.48) or had prolong hospital stay (40±6.42), again emphasizing the prognostic significance of hs-CRP [Table/Fig-7].
Comparison of outcome in SBP and non-SBP.
| SBP(n=50) | Non SBP(n=50) | p-value |
|---|
| Death | 12 (24.0%) | 5 (10.0%) | Z=2.63;p<0.05 |
| Mean Ascitic Fluid Hs-CRP Level Who Died | 80.20±13.65 | 44.56±8.48 | t98 = 15.68; p=0.00001 |
| Hospital Stay More Than 2 Week | 15 (30.0%) | 9 (18.0%) | Z=1.99;<0.05 |
| Hs-CRP Level in Hospital Stay More Than 2 Week | 74±11.75 | 40 ±6.42 | t98 = 18.56;p=0.00001 |
Discussion
Present study was conducted with the objective of establishing the role of hs-CRP as a prognostic indicator in patients with Spontaneous Bacterial Peritonitis (SBP) and to compare its level in patients with SBP and patients with sterile ascites.
Aetiology
Literature shows that in India most cases of cirrhosis are due to alcohol and viral hepatitis B and C [12]. These results were in accordance with the present study [Table/Fig-3].
Symptoms and Signs
In our study the predominant symptoms were abdominal pain (76%) followed by fever (72%) and tenderness of abdomen on palapation (64%). Syed VA et al., in his study found that the most common presenting symptoms were UGI bleeding (75%) followed by pain abdomen (65%) [13]. Fever was found in 45%. Minhas et al., reported fever 54%, pain abdomen 57% and Hepatic encephalopathy 67% [10]. Completely asymptomatic cases have been reported between 14%-100%. Great variation in symptoms and signs has been reported in different studies.
Culture
In our study most common organism isolated was E.coli (38%) followed by Kliebsiella (16%), Strep. Viridians (8%), and Enterococcus (4%) and 34% cases had CNNA [Table/Fig-4].
Serum Creatinine
The mean Serum creatinine in SBP patients in our study was significantly higher than the controls [Table/Fig-2]. Increased levels of seum creatinine in cases of decompensated cirrhosis can occur due to a number of factors like excessive diuretic therapy, haematemesis, intravascular volume depletion, large volume paracentesis, shock and most importantly development of hepato renal syndrome. Infection is a well known entity that causes rise in serum creatinine in cases of cirrhosis of liver. Other studies had similar results [14].
International Normalized Ratio
In this study INR of cases was significantly raised than the controls [Table/Fig-2]. Similar to our findings increase prothrombin time was seen in cases of SBP in a study [15].
Child Pugh Score
Child’s score was originally designed for predicting the prognosis of cases of cirrhosis with portal hypertension who underwent surgery, where portocaval shunt was a trade off. Child’s score included two continuous variables (bilirubin and albumin) and three discrete variables (ascites, encephalopathy, and nutritional status). The discrete variables were included because they had significant influence on the prognosis of these patients. In this view we studied the influence of SBP and Child’s criteria. Studies have shown that infection in cirrhosis co relates adversely with child-pugh scores [16,17].
In our study, 78% of patients with SBP were in Child’s class C or B, (p< 0.05) compared to controls) suggesting that SBP adds to the additional burden for the prognosis of cirrhosis. This was in agreement with other studies that showed SBP developed with more advanced liver disease and adversely affected the outcome of the patients [18].
It would be interesting to discuss that the original Child Pugh’s criteria only includes ascitis as one of the factors, whether SBP should be also added as an additional factor for estimating prognosis in cases of cirrhosis should be pondered upon.
Ascitic Highly Sensitive CRP (hs-CRP)
In our study the mean hs-CRP level of the patients with SBP (77.20 ± 11.65) was significantly higher than that of the patients without spontaneous bacterial peritonitis (42.50 ± 7.48) (p<0.01) [Table/Fig-2,5]. But it was also interesting to note that the mean hs-CRP in the control group were also more than the mean standard normal range of hs-CRP (10 mg/l) in active inflammation. This could be again explained by the fact that there is existence of continuous inflammation in decompensated cirrhosis. Evidence suggests the active role of TNF-α, Cytokine storm, IL 6, Toll like receptor activity all are involved in the active inflammatory process, so even decompensated cirrhosis can also increase the inflammatory markers. SBP adds to the existing inflammatory burden leading to still higher levels of inflammatory markers like hs-CRP.
In this study the mean Hs-CRP levels at 5th day after initiation of antibiotic therapy or, discharge were significantly lower, compared to that of before initiation of antibiotic therapy (p<0.01) [Table/Fig-6]. This suggests that early suspicion, detection and treatment of SBP do contribute to decrease the inflammation burden and overall prognosis of SBP patients. Guler K et al., studied serum hs-CRP level in cases of SBP and compared serum hs-CRP levels two days after standard antibiotic treatment [19]. There was statistically significant decrease in the mean serum hs-CRP level two days after antibiotic therapy.
Prognosis and Out Come
Hospital stay was significantly longer in patients of SBP (12.0 vs 5.0 days) while poor outcome was also significantly associated with patients of SBP (24% vs 10% deaths).
The mean ascetic fluid hs-CRP level in the patient who died (80.20±13.65) and in the patient who had prolong hospital stay more than two weeks (74±11.75) was also significantly higher than the mean ascetic fluid hs-CRP level of the controls who died (44.56±8.48) or had prolong hospital stay (40 ±6.42), again emphasizing the prognostic significance of hs-CRP [Table/Fig-7].
Conclusion
In the present study, we conclude that greater ascitic fluid hs-CRP levels in SBP poorly correlate with the prognosis of the patients with cirrhosis. Prompt early detection and treatment is important for a favourable outcome. Ascitic fluid hs-CRP can be considered as a surrogate prognostic marker in cases of SBP.
χ2 = 88.57; p=0.0001; S-Significant