Introduction
A popular rat model for hypoperfusion ischemic brain injury is bilateral common carotid artery occlusion (BCCAO). BCCAO surgery when performed in varying geographical locations and during different seasons of the year is reported to have variable mortality rates. Studies have also documented the diminishing influence of Ketamine-Xylazine (KT-XY) on thermoregulatory functions in rodents.
Aim
To explore the impact of seasonal variant temperatures and laboratory room ambient temperatures on mortality of rats following BCCAO surgery.
Materials and Methods
The study has two parts:
1 The first part is an analysis of a three year retrospective data to explore the association between the geographical season (hot summer and cold winter) induced laboratory room ambient temperature variations and the mortality rate in KT-XY anaesthetized BCCAO rats.
2. The second part investigated the effect of conditioned laboratory room ambient temperature (CAT) (23-250C) in KT-XY anaesthetized BCCAO group of rats. Rats were divided into 4 groups(n =8/group) as-Normal control, BCCAO and Sham BCCAO where they were all exposed to unconditioned ambient temperature (UCAT) during their surgery and postoperative care. And finally fourth group rats exposed to CAT during the BCCAO surgery and postoperative care.
Results
Pearson’s chi-square test indicates a significantly high association (p<0.006) between post-BCCAO mortality and hot season of the year. CAT during the hot season reduced the mortality rate (24% less) in post- BCCAO rats compared to the rats of UCAT.
Conclusion
Despite seasonal variations in temperature, conditioning the laboratory room ambient temperatures to 23–250C, induces hypothermia in KT-XY anaesthetized ischemic brain injured rodents and improves their survival rate.
Ischemic heart disease, Seasonal variations, Post-operative care
Introduction
Stroke is defined as; “rapidly developing clinical signs of focal or global disturbance of cerebral function, with symptoms lasting 24 hours or longer or leading to death, with no apparent cause other than of vascular origin [1]. Stroke is the second leading cause of death in the world next to ischemic heart disease [2]. It is also one of the leading causes for disability in the world with one-third of the surviving stroke patients being dependent on others for their activities of daily living [3]. Previous studies have documented seasonal variations in the stroke incidence and mortality in humans hospitalized for stroke treatment. United States (US) health registry has reported an increased number of hospitalizations and deaths occur in hot season, which is attributed to elevated ambient temperatures [4]. Animal models of ischemic stroke serve as useful tool to study mechanisms of ischemic cerebral injury and to develop novel anti-ischemic or neuroprotective strategies. Better survival rate, satisfactory recovery from surgery and reproducible behavioural testing have made rodents the most preferred species to explore novel therapeutic strategies on stroke [5]. Conventionally experiments with rodent stroke models have either used permanent or temporary occlusion of intracranial (e.g. middle cerebral artery) or extracranial (e.g. vertebral basilar or common carotid arteries) arteries associated with hypotension or hypoxia [6].
Rat models with occlusion of extra cranial vessels are frequently used to study cerebral hypo-perfusion induced partial or global ischemia. Bilateral Common Carotid Artery Occlusion (BCCAO) Wistar rat model induces chronic cerebral hypo perfusion ischemic brain injury in a manner similar to what is seen in hypo-perfusion of aging brain associated with vascular dementia [7]. A major advantage of this model is that it can be suitably modified to study the acute and chronic changes subsequent to ischemic brain injury. BCCAO rats undergo a brief period of acute ischemic phase followed by a phase of chronic cerebral hypo-perfusion which allows the investigator to explore and study new therapeutic strategies aimed at enhancing both the acute and chronic phase of stroke management [6].
Despite the several advantages of this BCCAO surgery model in rats, very few neuroscientists choose to work on this model due to its high (44%) and variable mortality rate [8,9]. Both in on-going studies as well as our earlier experiments using BCCAO model [10], a seasonal variability in mortality rate post-surgery was observed. The percentage of death in BCCAO rat model was highest during hot season of the year (March-May) in Manipal, Udupi District, a coastal town in the Southern part of India (74°53’ East, 12°55’ North). Bhat S et al., have also reported that the high ambient temperature and humidity of the hot season brings neuroendocrine imbalance and oxidative stress in female Wistar rats [11].
Commonly used anaesthetics like Ketamine–Xylazine (KT-XY), interferes with body’s haemodynamic and thermoregulatory mechanisms and can reduce the core body temp to 32.30±0.090C, and the effect can persist even 150 min after the induction of anaesthesia [12,13]. Studies have documented that rodents anaesthetized with KT-XY develops hypothermia postoperatively and is reported to be one of the frequent causes for mortality and morbidity following any invasive surgical procedure. Additionally the standard postoperative care recommended for all rat models is prevention of hypothermia by increasing the ambient temperature of the procedure room, placement of a thermal blanket, careful and controlled use of incandescent or heat lamps during surgery and post-surgical recovery and administration of warmed subcutaneous or intraperitoneal fluids intra- and/or postoperatively [14,15].
No study till date has shown whether conditioning the ambient temperature to the desired level would reverse the post-stroke mortality rate and other abnormal changes in homeostasis. Although the benefits of maintaining a constant ambient room temperature is obvious, a systematic study to prove the advantages of the same in reducing mortality of postoperative BCCAO rats and other physiological variations is essential. In the present study post BCCAO rats were maintained in an isolated lab room without any air-conditioning facility to determine the role of lab room ambient temperature in preventing hypothermia and reducing mortality.
Additionally, research studies have also reported that hypothermia is a robust neuro-protectant that consistently shows functional recovery from brain injuries [16].
In this study, we have evaluated the effect of seasonal variation-induced changes in lab room ambient temperature on mortality rate of BCCAO rats and also explored the role of controlled lab room ambient temperature despite seasonal variations on survival of BCCAO rats.
Aim
To analyse the three year- retrospective experimental data of observed seasonal variation-induced lab room ambient temperature on the mortality rate of rats that had undergone BCCAO surgery and exposed to recommended standard postoperative care.
To explore the effect of conditioned ambient temperature despite seasonal variations in temperature contributing to mortality rate of rats that had undergone BCCAO surgery and exposed to recommended standard postoperative care.
Materials and Methods
The experimental design of this study has two parts.
Part-1: The first part of this study is an outcome of a retrospective three- year data (2011-2014) analysis to explore the association between the geographical season-induced ambient lab room temperature variations and the mortality rate in 8-10-month-old male Wistar rats that had undergone BCCAO surgery with standard recommended postoperative care. The data were analysed, correlated and interpreted for the mortality rate of BCCAO rats and sham BCCAO rats with the ambient temperature variations induced by different geographical seasons.
These experiments were carried out in the same set of months every year.
The first set of experiments were done between the months of March and May, 2011-2014 in Manipal, a coastal town in South India, which has a hot and humid climate between the months of March and May {Hot Season (HS)} with average temperatures ranging from 33-37°C and an average humidity ranging from 60-70%.
The second set of experiments were conducted between June and February (maximally during July-August & December-February), 2011-2014. In Manipal, humid and cool climate is recorded between June and February {Cold Season (CS)} with average temperatures ranging from 25-29°C and an average humidity ranging from 80-90%. There is no distinct winter season in this region but southwest monsoons bring heavy rainfall during the months of June to September with an average rainfall 4136 mm/year (c/f: www.accuweather.com).
The data from the first set of experiments (March- May, 2011-2014) were grouped together. They were further sub-classified as HS-BCCAO group of rats (n=37) and HS-sham BCCAO group of rats (n=35). During HS these rats underwent either BCCAO surgery or sham BCCAO surgery (surgical procedure same as that of BCCAO, except for carotid artery occlusion). The average experimental laboratory room ambient temperature during HS ranged from 33-36°C which was slightly less than the outside atmospheric temperature. Likewise, the average humidity of the room matched with that of the outside atmospheric air and was found to range from 60-70%.
The data from the second set of experiments (June-February, 2011-2014) were grouped together and were further sub-classified as CS-BCCAO group of rats (n=39) which underwent BCCAO and CS- Sham BCCAO group of rats (n=28) underwent Sham BCCAO surgery during CS. The average experimental laboratory room ambient temperature during CS ranged from 26-27°C which was slightly more than the outside atmospheric temperature and the average humidity of the room matched with that of the atmosphere and was found to range from 80-90%.
Part-2: The second part of the study was planned and executed between March and May, 2014. This study was done to further investigate the observations recorded during part-1 experimental findings. Ten months-old male Wistar rats were divided into four groups (n =8/group) as:
NC = Normal control Wistar rats reared in unconditioned ambient temperature.
UCAT-BCCAO = Wistar rats, reared in unconditioned ambient temperature (UCAT), were subjected to BCCAO surgery and postoperative care was given for 32 days in the same environment.
CAT-BCCAO = Conditioned ambient temperature (CAT) BCCAO group-Conditioned ambient temperature (23-250C) was provided in an air-conditioned facility during the BCCAO surgery and postoperative period for 32 days.
Sham BCCAO = Rats were reared and subjected to sham BCCAO surgical procedure and maintained postoperatively for 32 days in UCAT.
Standard food pellets and water was provided ad libitum to all animals throughout the study. Standard hygienic conditions were maintained in the animal facility and the rats were exposed to proper light and dark cycle. This study was approved by Institutional Animal Ethical committee of KMC, Manipal (IAEC/KMC/66/2010-2011) and carried out as per the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India, guidelines.
Bilateral Common Carotid Artery Occlusion Surgery Induced Brain Injury
Preoperatively the Wistar rats were starved without food for 12 hour and water was allowed ad-libitum. Chronic cerebral hypoperfusion ischemic brain injury was induced in Wistar rats as described below. Atropine (130 mg/Kg Bodywt IP) and gentamycin (4.4 mg/Kg body weight) were administered as pre-anaesthetic medication and the rats were anaesthetized with ketamine (50mg/kg body weight IP) and xylazine (5mg/kg body weight IP) cocktail. Midline incision was made extending between mandible and manubrium sterni. Skin and superficial fascia were cleared and retracted using blunt forceps. Common carotid arteries on the both sides were identified and gently separated from vagus nerve using a glass rod. The carotid arteries were double ligated with silk suture just before the carotid bifurcation and all the tissues were closed in the layers. Sham BCCAO surgery was similar to the actual BCCAO surgery with the exception of carotid artery occlusion. Post-surgery, all the rats were injected with 2 ml (IP) of dextrose normal saline to prevent dehydration and hypoglycaemia [10]. Appropriate standard postoperative care (infrared lamp to prevent hypothermia) was provided along with proper surgical dressing using betadine solution (Povidone ointment USP). During the 15 days of recovery, the rats were maintained on a special platform designed to allow easy accesses to food and water [14].
Statistical Analysis
Data obtained on mortality-rate of Wistar rats from the three-year retrospective study was analysed using SPSS v16. The relation between the mortality rates of rats that underwent BCCAO surgeries during different seasons (HS-BCCAO Vs CS-BCCAO) were analysed using Pearson’s Chi-square test. Similarly the mortality rate in the group of rats that had undergone BCCAO surgery compared with sham BCCAO group of rats during both seasons were analysed by using the same Pearson’s Chi-square tests. The p≤0.05 was considered as statistically significant. The mortality rate between the four groups in the second part of the experiments were compared and reported in percentage.
Results
Analysis of mortality-rate among Wistar rats from the three-year retrospective study indicates that the mortality rate of Wistar rats following BCCAO surgery ranged between 13.5 - 60% which is shown in [Table/Fig-1].
Post-BCCAO mortality rate of Wistar rats during HS (March-May, 2011-2014) and CS (June-February, 2011-2014). BCCAO- Bilateral common carotid artery occlusion; HS- Hot season; CS- Cold season
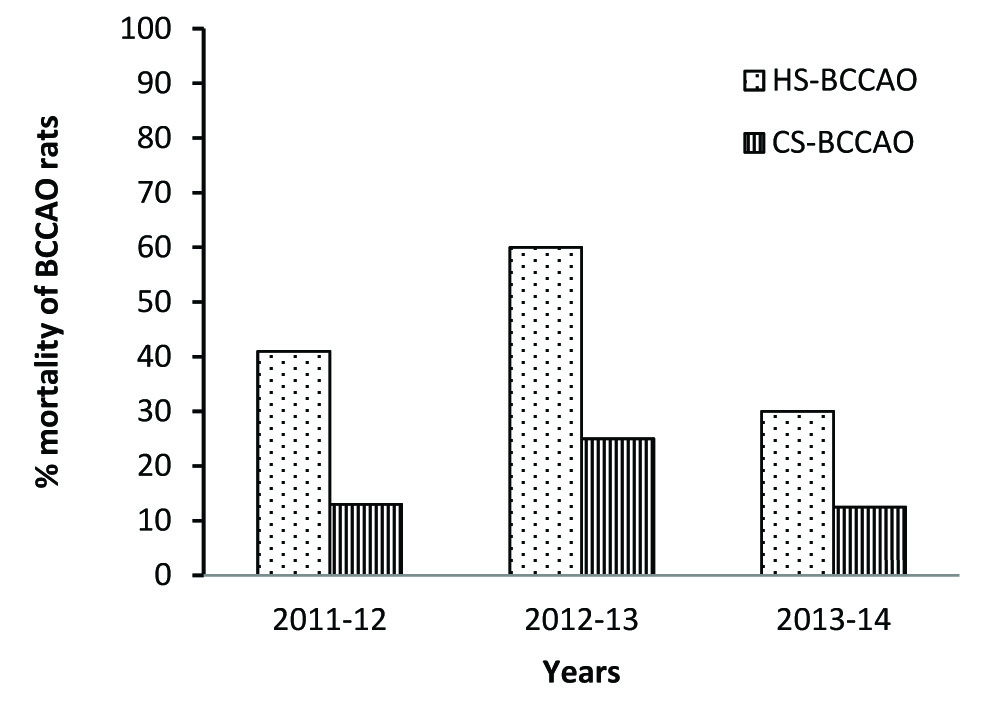
On categorizing the results in terms of season and statistically analysed a significantly higher mortality rate {σ2 = 8.65, df =1; 2 sided p < 0.006} was observed in HS-BCCAO rat groups when compared to CS-BCCAO rat groups as shown in [Table/Fig-2]. Majority of rats that died subsequent to BCCAO surgery during HS consistently had episodes of severe epileptic seizures and respiratory distress before their death. Very few HS-BCCAO rats, that suffered seizures, recovered fully and survived BCCAO surgery. Surprisingly the mortality rate of the two groups of Sham BCCAO rats during the varying seasons did not show any significance {σ2 =0.69, df=1; 2 sided p<0.62} as shown in [Table/Fig-2]. In addition, a significant association was observed between mortality rate during HS {σ2 =13.56, df=1; 2 sided p <0.0001} and BCCAO rats compared with sham BCCAO. But there was no significant association between mortality rate during CS {σ2 =2.75, df=1; 2 sided p <0.225} and BCCAO rats compared with sham BCCAO as shown in [Table/Fig-2].
Retrospective analysis of mortality rate in post BCCAO Wistar rats and post sham-BCCAO rats during hot and cold seasons of 2011-2014 in Manipal.
| Groups | Hot Season | Cold Season |
|---|
| Surviving ratsn(%) | Dead ratsn(%) | Total ratsn(%) | Surviving ratsn(%) | Dead ratsn(%) | Total ratsn(%) |
|---|
| Post BCCAO | 20 (54.1%) | 17 (45.9%) | 37 (100%) | 33 (84.6%) | 6 (15.4%)* | 39 (100%) |
| Post Sham BCCAO | 32 (91.4%) | 3# (8.6%) | 35 (100%) | 27 (96.4%) | 1 (3.6%)@,,$ | 28 (100%) |
*Fisher exact test 2 sided p<0.006. Post stroke mortality rate of HS BCCAO compared with CS BCCAO.
@Fisher exact test 2 sided p<0.622. Post stroke mortality rate of HS sham-BCCAO compared with CS sham-BCCAO.
#Fisher exact test 2 sided p<0.001. Post stroke mortality rate of HS BCCAO compared with HS sham-BCCAO.
$Fisher exact test 2 sided p<0.225. Post stroke mortality rate of CS BCCAO compared with CS sham-BCCAO.
BCCAO- Bilateral common carotid artery occlusion; HS-Hot season (March-May,2011-2014); CS- Cold season (June-February, 2011-2014).
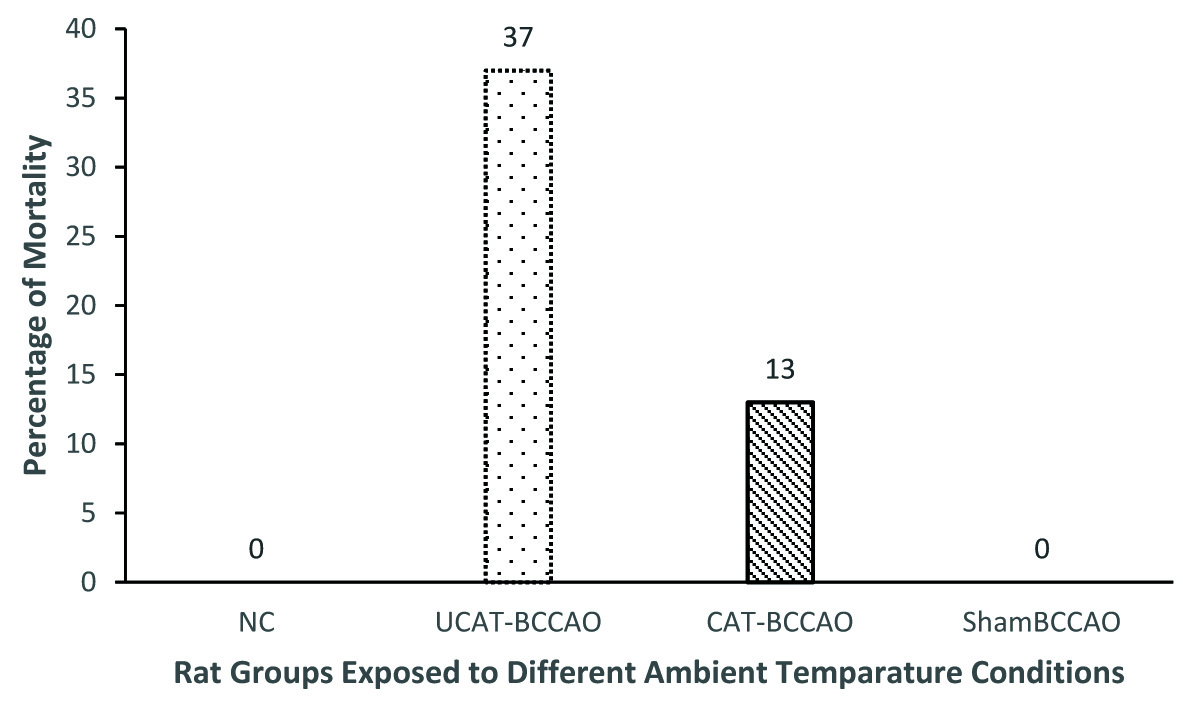
Alternately, the mortality rates of UCAT-BCCAO and CAT-BCCAO in part-2 experiments are 37% and 13% respectively. None of the rats died (0%) in the sham BCCAO group. Though there is no significant difference statistically in the mortality rate between rats from UCAT-BCCAO and CAT-BCCAO groups, there is a definite trend in reduction of mortality rate (24% less) in CAT-BCCAO compared to UCAT-BCCAO group of rats as shown in [Table/Fig-3].
Comparison of mortality rate of rats under CAT and UCAT during HS (March-May 2014). BCCAO- Bilateral common carotid artery occlusion; HS- Hot season; CAT – conditioned ambient temperature; UCAT – unconditioned ambient temperature
Discussion
The results of the three-year retrospective study on BCCAO surgery indicate that there is a significantly high and variable mortality rate in Wistar rats subsequent to the surgery. Moreover, the survival rate of rats that have undergone BCCAO surgery during CS is relatively better compared to the same during HS. Analysis of data on this study related to outcome of BCCAO and Sham BCCAO surgery during HS indicates that the high mortality rate in BCCAO rats could be due to accentuation of acute phase of neuronal injury by unconditioned hot ambient temperature. A similar phenomenon is also reported in a United States human epidemiological study, showing a significant increase in the mortality rate in patients hospitalized for their pre-existing illness (Cardiovascular, respiratory, and/or cerebrovascular diseases) during hot summer seasons and elevated ambient temperature [17,18]. The temperature variations in stroke rats have shown to critically influence the neuro-pathological outcome [19]. Surprisingly in our study, 84% of rats that underwent Sham-BCCAO surgery survived since any variation in ambient temperature do not affect normal neural cells in these rats. Hence, there was no significant difference in the survival rate among these groups of rats during different seasons of the year.
In the second part of the experiment, BCCAO group of rats that underwent surgery and postoperative care in conditioned ambient temperatures (23-250C) showed better survival rate compared to the UCAT-BCCAO group of rats during the hot humid months of March- May despite use of recommended standard postoperative care of exposing post BCCAO rats to infrared lamp. Hypothermia induced by both KT-XY anaesthesia as well as conditioned cold ambient temperature during the acute phase of ischemic brain injury possibly synergistically attributes to the improved survival rate in CAT-BCCAO rats post-surgery. It has been well documented that use of KT-XY can interfere with thermoregulatory mechanisms and can result in postoperative hypothermia following any surgical procedure in rodents [20]. The hypothermia in rats in our experiments was also achieved by exposing the animal to low ambient temperatures maintained between (23-250C) using an air conditioning facility during the intra-ischemic (during BCCAO surgery) and post-ischemic periods.
A recent study has shown that the synergetic neuro-protective effect of allopregnanolone and other GABA-A receptor agonists with hypothermia in stroke rats are predominately due to spontaneously evolving postoperative hypothermia in brain injured rats when No attempts were made to maintain the rats core body temperature with any other external measures [21]. Extensive research on the effects of rat body temperature modulation clearly reveal that when compared to normo-thermic rats, the rats exposed to hyperthermia (rats subjected to experimental temperature modulation so that brain temperature is equilibrated 38-400C) particularly during the intra-ischemic and post ischemic period exhibited increased histopathological damage and a greater mortality in the first 24 hours [19]. Previous studied have reported post-ischemic hyperthermia aggravates oxidative stress, glutamate excitotoxicity, leucocyte infiltration and disruption of blood brain barrier in ischemic brain injured rats [22–24]. Experimental research on rats have documented that ischemic brain injured rats with intra-ischemic hyperthermia (39°C) have greater damage to CA1 hippocampal neurons and exhibit higher mortality at 24 hours compared to the rats exposed to normal temperature [19]. Earlier studies from our lab by Bhat S et al., also have shown us that unconditioned ambient temperature and humidity during hot weather season can increase plasma cortisol levels and oxidative stress in rats [11]. Harmon et al., have reported a reduction in antioxidant activity of plasma in mid lactating cows when they are exposed to chamber temperatures of 29.5°C [25].
Studies done on pilots who were exposed to extremes of temperature have shown enhanced lipid peroxidation and dec-reased antioxidant ability in human erythrocytes [26]. Following deprivation of blood supply, the injured ischemic brain exhibits three zones, (i) the central ischemic core zone, (ii) the surrounding ischemic penumbra zone and (iii) the external zone of normal tissue. In the central infarct region the neurons are dead and necrosed, whereas the ischemic penumbra shows isoelectric electroencephalogram but morphologically normal neurons. The ischemic penumbra is also a frequent site for apoptosis and delayed neuronal cell death. Studies have shown that hyperthermia enhances the chances of penumbra region going in for a frank infarction. Hyperthermia accelerates the evolution of ischemic necrosis in both cerebral cortex and striatum [27].
Research over the past decade has shown controlled hypothermia to be an effective neuro-protective strategy and its protective effects are attributed to its ability to cause a reduction in the metabolic demands and to minimize the oxidative stress and acidosis of the hypo-perfused regions of the brain [28–32]. Previous studies have shown that inducing intra-ischemic and post-ischemic hypothermia can minimize glutamate excitotoxity and reduces the release of dopamine by 60% thus reducing neuronal injury [33]. Research on neonatal rat models of severe hypoxic ischemic encephalopathy showed that post-ischemic hypothermia with a target temperature of 33°C or less decreased infarct volume and improved sensorimotor functions [34]. In the present study too we found that the mortality rate post-BCCAO surgery was significantly low in the CS-BCCAO group of rats when compared with HS-BCCAO group of rats. This could be due to reduced ambient and niche temperature reducing the brain temperature of BCCAO rats post-surgery during CS. Azzimondi G et al., in his prospective study on human patients admitted for acute stroke brain injury suggests that the high temperature of 38.30C during first seven days is an independent risk-factor for poor prognosis in stroke recovery [35].
Conclusion
Exposing post ischemic brain injured rats to warm/hot ambient temperature by infrared lamps, heating pad as part of standard postoperative care as well as un-conditioned lab room ambient temperature that is influenced by hot seasonal variations increases mortality and morbidity of rats. It has been well documented that intra and post ischemic hyperthermia accentuates the events of ischemic cascade and can cause the zone of severe neuronal injury to extend beyond the core infarct area to involve regions of ischemic penumbra resulting in a more severe disability or a higher mortality among the rat models of ischemic brain injury. Our study confirms the fact that the high mortality rate of rats following BCCAO surgery during HS is due to hot and humid unconditioned lab room ambient temperatures observed in our experimental study.
*Fisher exact test 2 sided p<0.006. Post stroke mortality rate of HS BCCAO compared with CS BCCAO.
@Fisher exact test 2 sided p<0.622. Post stroke mortality rate of HS sham-BCCAO compared with CS sham-BCCAO.
#Fisher exact test 2 sided p<0.001. Post stroke mortality rate of HS BCCAO compared with HS sham-BCCAO.
$Fisher exact test 2 sided p<0.225. Post stroke mortality rate of CS BCCAO compared with CS sham-BCCAO.
BCCAO- Bilateral common carotid artery occlusion; HS-Hot season (March-May,2011-2014); CS- Cold season (June-February, 2011-2014).
[1]. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke AssociationStroke; A Journal of Cerebral Circulation 2013 44(7):2064-89. [Google Scholar]
[2]. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Heart disease and stroke statistics–2015 update: a report from the American Heart AssociationCirculation 2015 131(4):e29-322. [Google Scholar]
[3]. Warlow CP, Epidemiology of strokeLancet 1998 352(Suppl 3):Siii1-4. [Google Scholar]
[4]. Basu R, Relation between Elevated Ambient Temperature and Mortality: A Review of the Epidemiologic EvidenceEpidemiologic Reviews 2002 24(2):190-202. [Google Scholar]
[5]. Casals JB, Pieri NC, Feitosa ML, Ercolin AC, Roballo KC, Barreto RS, The use of animal models for stroke research: a reviewComparative Medicine 2011 61(4):305-13. [Google Scholar]
[6]. Farkas E, Luiten PG, Bari F, Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseasesBrain Res Rev 2007 54(1):162-80. [Google Scholar]
[7]. Rizzi L, Rosset I, Roriz-Cruz M, Global epidemiology of dementia: Alzheimer’s and vascular typesBio Med Research International 2014 2014:908915 [Google Scholar]
[8]. Melo MC, Gadelha D, Mascena GV, Oliveira TK, Brandt CT, Learning and survival memory undergoing a permanent bilateral carotid ligation in ratsActa cirurgica brasileira / Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia 2013 28(2):102-05. [Google Scholar]
[9]. Kim SK, Cho KO, Kim SY, White Matter Damage and Hippocampal Neurodegeneration Induced by Permanent Bilateral Occlusion of Common Carotid Artery in the Rat: Comparison between Wistar and Sprague-Dawley StrainKorean J Physiol Pharmacol 2008 12(3):89-94. [Google Scholar]
[10]. Sivakumar G, Vidyadhara D, Reddy S, Rajesh T, Babu MR, Rao KM, Prophylactic combined supplementation of choline and docosahexaenoic acid attenuates vascular cognitive impairment and preserves hippocampal cell viability in rat model of chronic cerebral hypoperfusion ischemic brain injuryInt J Basic Clin Pharmacol 2015 4(3):522-30. [Google Scholar]
[11]. Bhat S, Rao G, Murthy KD, Bhat PG, Seasonal variations in markers of stress and oxidative stress in ratsIndian journal of clinical biochemistry: IJCB 2008 23(2):191-94. [Google Scholar]
[12]. Rodrigues SF, de Oliveira MA, Martins JO, Sannomiya P, de Cassia Tostes R, Nigro D, Differential effects of chloral hydrate- and ketamine/xylazine-induced anaesthesia by the s.c. routeLife Sciences 2006 79(17):1630-37. [Google Scholar]
[13]. Chaves AA, Weinstein DM, Bauer JA, Non-invasive echocardiographic studies in mice: influence of anaesthetic regimenLife Sciences 2001 69(2):213-22. [Google Scholar]
[14]. Animal Care & Use Program: DUKE UNIVERSITY & MEDICAL CENTER; 2011 [cited 2015 15th June]. Available from: http://vetmed.duhs.duke.edu/Guidelines for Post Op Rodent Care. html# [Google Scholar]
[15]. Ljubisavljevic MR, Javid A, Oommen J, Parekh K, Nagelkerke N, Shehab S, The effects of different repetitive transcranial magnetic stimulation (rTMS) Protocols on cortical gene expression in a rat model of cerebral ischemic-reperfusion injuryPLoS one 2015 10(10):e0139892 [Google Scholar]
[16]. Yenari MA, Hemmen TM, Therapeutic hypothermia for brain ischemia: where have we come and where do we go?Stroke; A Journal of Cerebral Circulation 2010 41(10 Suppl):S72-74. [Google Scholar]
[17]. Basu R, High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008Environmental Health: a Global Access Science Source 2009 8:40 [Google Scholar]
[18]. Hajat S, Kosatky T, Heat-related mortality: a review and exploration of heterogeneityJournal of Epidemiology and Community Health 2010 64(9):753-60. [Google Scholar]
[19]. Kim Y, Busto R, Dietrich WD, Kraydieh S, Ginsberg MD, Rosenblum WI, Delayed Postischemic Hyperthermia in Awake Rats Worsens the Histopathological Outcome of Transient Focal Cerebral IschemiaStroke; a Journal of Cerebral Circulation 1996 27(12):2274-81. [Google Scholar]
[20]. Picollo C, Serra A, Levy R, Antonio E, Santos Ld, Tucci P, Haemodynamic and thermoregulatory effects of xylazine-ketamine mixture persist even after the anaesthetic stage in ratsArquivo Brasileiro de Medicina Veterinária e Zootecnia 2012 64(4):860-64. [Google Scholar]
[21]. Spratt NJ, Tomkins AJ, Pepperall D, McLeod DD, Calford MB, Allopregnanolone and its precursor progesterone do not reduce injury after experimental stroke in hypertensive rats - role of postoperative temperature regulation?PloS one 2014 9(9):e107752 [Google Scholar]
[22]. Williams WM, Lu ST, Del Cerro M, Michaelson SM, Effect of 2450 MHz microwave energy on the blood-brain barrier to hydrophilic molecules. D. Brain temperature and blood-brain barrier permeability to hydrophilic tracersBrain research 1984 319(2):191-212. [Google Scholar]
[23]. Dietrich WD, Halley M, Valdes I, Busto R, Interrelationships between increased vascular permeability and acute neuronal damage following temperature-controlled brain ischemia in ratsActa Neuropathol 1991 81(6):615-25. [Google Scholar]
[24]. Takagi K, Ginsberg MD, Globus MY, Martinez E, Busto R, Effect of hyperthermia on glutamate release in ischemic penumbra after middle cerebral artery occlusion in ratsThe American journal of Physiology 1994 267(5 Pt 2):H1770-76. [Google Scholar]
[25]. Harmon R, Lu M, Trammel D, Smith B, Influence of heat stress and calving on antioxidant activity in bovine bloodJ Dairy Sci 1997 80(Suppl 1):264 [Google Scholar]
[26]. Hao WW, Qin SZ, Yu QF, Li MG, Ma GX, Zhao H, Effects of heat and noise environments on lipid peroxidation erythrocyte membrane in pilotsSpace Medicine & Medical Engineering 2000 13(1):52-54. [Google Scholar]
[27]. Dietrich WD, Busto R, Valdes I, Loor Y, Effects of normothermic versus mild hyperthermic forebrain ischemia in ratsStroke; a Journal of Cerebral Circulation 1990 21(9):1318-25. [Google Scholar]
[28]. Dezena RA, Colli BO, Carlotti Junior CG, Tirapelli LF, Pre, intra and post-ischemic hypothermic neuroprotection in temporary focal cerebral ischemia in rats: morphometric analysisArquivos de Neuro-Psiquiatria 2012 70(8):609-16. [Google Scholar]
[29]. Fleming R, Acid-base balance of the blood in dogs at reduced body temperatureAMA Archives of Surgery 1954 68(2):145-52. [Google Scholar]
[30]. Deterling RA, Nelson E, Bhonslay S, Howland W, Study of basic physiologic changes associated with hypothermiaAMA Archives of Surgery 1955 70(1):87-94. [Google Scholar]
[31]. Hagerdal M, Harp J, Nilsson L, Siesjo BK, The effect of induced hypothermia upon oxygen consumption in the rat brainJournal of Neurochemistry 1975 24(2):311-16. [Google Scholar]
[32]. Rosomoff HL, Holaday DA, Cerebral blood flow and cerebral oxygen consumption during hypothermiaThe American Journal of Physiology 1954 179(1):85-88. [Google Scholar]
[33]. Busto R, Dietrich WD, Globus MY, Ginsberg MD, The importance of brain temperature in cerebral ischemic injuryStroke; a Journal of Cerebral Circulation 1989 20(8):1113-14. [Google Scholar]
[34]. Lee BS, Woo CW, Kim ST, Kim KS, Long-term neuroprotective effect of postischemic hypothermia in a neonatal rat model of severe hypoxic ischemic encephalopathy: a comparative study on the duration and depth of hypothermiaPediatric Research 2010 68(4):303-08. [Google Scholar]
[35]. Azzimondi G, Bassein L, Nonino F, Fiorani L, Vignatelli L, Re G, Fever in acute stroke worsens prognosis. A prospective studyStroke; a Journal of Cerebral Circulation 1995 26(11):2040-43. [Google Scholar]