Introduction
Chronic Myelogenous Leukaemia (CML) is a malignant clonal disorder of haematopoietic stem cells prompting monstrous extension of myeloid lineage cells [1]. The fate of CML is to advance from a benign chronic phase into the fatal blast crisis between 3 and 5 years. Advancement of CML is connected with a particular chromosomal translocation known as the Philadelphia (Ph) chromosome that is recognizable over the span of the disease [2].
Apoptosis is a physiological cell suicide program that is basic for the improvement and maintenance of healthy tissues. Failure to trigger the cellular suicide program not only inclines to development of malignancies, but also expands resistance of tumours to anticancer drugs and irradiation [3]. Therefore cancer can be partially attributed to defects in the regulation of apoptotic cell death. Curcumin, also known as diferuloylmethane, a natural component of the rhizome of Curcuma longa has risen as a standout amongst the most intense chemopreventive and anticancer agents. Many studies concluded that curcumin induce cell cycle arrest and/or apoptosis in human cancer cell lines derived from variety of solid tumours including colorectal, lung, breast, pancreatic and prostate carcinoma, amongst others [4]. Curcumin has been found to moderate tumour cell development [5] and angiogenic process [6] invitro and in rodent experiments. Additionally, curcumin appears to induce apoptosis in cancer cells invitro without harming the healthy ones. Curcumin as anticarcinogenic agent, elevates intracellular ROS and prompts loss of mitochondrial membrane potential and apoptosis in leukaemia cells [7]. Owing to its anticarcinogenic property, curcumin not only can be used as chemotherapeutic agent but also been suggested for chemoprevention. Among various molecular mechanisms of curcumin, its anticancer properties halts the growth of many types of cancer cells at different stages of cancer progression is most likely due to its potential to act on multiple targets [8–10].
Curcumin exerts its effects via modulation of several cellular receptors (EGFR and HER2), signal transcription factors (NF-kB, AP-1, Egr-1, b-catenin, and PPAR-c), various oxygenases {cycloxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX)}, inducible nitric oxide synthase (iNOS), cytokines (TNF, IL-1, IL-6, chemokines), cell cycle proteins (cyclin D1p21), as well as cell surface adhesion molecules [11].
Several preclinical and clinical studies suggest that curcumin may represent a novel strategy to treat cancer patients [3]. However, the invivo application of curcumin has been constrained for its low potency and inadmissible bioavailability [12], which necessitates the application of new formulation solutions and amalgamation of novel curcumin analogs with enhanced pharmacological properties, while holding a comparable wellbeing profile. It can inhibit tumour metastasis, invasion and angiogenesis [13–17].
Tumour necrosis factor Related Apoptosis-Inducing Ligand (TRAIL) has been proposed as a novel anticancer agent because of its ability to induce apoptosis in tumour cells. Several members of tumour necrosis factor family such as Fas ligand, tumour necrosis factor (TNF)-, TRAIL are shown to induce apoptosis in susceptible cells [18,19]. TRAIL induces apoptosis in a wide variety of transformed cells [20].
TRAIL act as an anti-cancer therapeutic agent both invitro and invivo, the wide expression of TRAIL and TRAIL-Rs in various normal tissues recommends that the physiological role of TRAIL is more typical than induction of apoptosis in cancer cells [21]. TRAIL is not cytotoxic on normal clonogenic haematopoietic progenitors [22–24]. It has been demonstrated that it specifically affects erythroid development by targeting on immature erythroblasts [24–27] and acts in a stage of different particular manner, as a negative regulator of normal erythropoiesis [25]. Expanded expression of TRAIL at the bone marrow level is liable to hinder erythropoiesis and add to the level of iron deficiency, that become the major clinical component of Myelodysplastic syndrome (MDS) [28].
In this study, we investigated whether curcumin alone or in combination can enhance TRAIL-mediated apoptosis in chronic myeloid leukaemic cells.
Materials and Methods
This invitro study was done in Molecular laboratory, Department of Zoology, Lucknow university, for the period of 3 years.
Reagents: Curcumin was procured from Sigma Chemical Co., USA. A 10 mM solution of curcumin was prepared in DMSO, and all test concentrations were prepared by diluting the stock solution in tissue culture medium. TNF-related apoptosis-inducing ligand (TRAIL) was procured from R&D Systems (Minneapolis, MN).
Cell Lines and Culture Conditions: The human myeloid cell line KCl-22 was purchased from National Centre for Cell Sciences (NCCS) Pune, India and cultivated in RPMI Media (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 0.2% sodium bicarbonate (Merck) and 1% (1x solution) of antibiotics (Invitrogen). The pH of the medium was adjusted to 7.4 with the help of 0.1N NaOH / 0.1 N HCl followed by filter sterilization using the Millipore filters of pore size 0.22 μm. The medium was then aliquoted into 100-200 ml and kept in CO2 incubator for at least two days to check the sterility of medium. Cultures were maintained at 37°C and 5% CO2 under high humid atmosphere. Medium was changed twice weekly and the cultures were split at a ratio of 1:5 once a week. Images were taken with the help of inverted microscope (Nikon ECLIPSE Ti-S, Japan)
Measurement of Cell Viability Assay: This activity is based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye reduction assay which gives an immediate relationship between the viable cells and absorbance. To evaluate the antiproliferative effect of curcumin, MTT assay was performed with some modification [29]. KCL22 cells (1x104) were seeded in each well of 96-well culture plate for overnight at 37°C and 5% CO2. Stock solutions of compound was prepared and diluted to obtain desired concentrations (10, 25, 50, 100 and 150 μM) in culture medium. This solution was then added to the wells as per experimental design. 10 μl of MTT (5 mg/ml of media without phenol red and serum) solution was added in each well after 24 and 48 hour of treatment, and plates were incubated for 3 h at 37°C until formazan blue crystal developed. Then, the supernatant was disposed off from each well and DMSO (100 μl) was added to solubilize formazan crystals for 10 min at 37°C. The absorbance was recorded at 540nm by a microplate reader (BIORAD-680). The percentage viability was calculated by using the formula
% Cell viability = {(OD of treated) (OD of control)} X 100
The plot of % cell viability versus CQ concentrations was used to compute the concentration lethal to 50% of the cells (IC50). The cellular morphological changes were observed under inverted phase contrast microscopy (Nikon ECLIPSE Ti-S, Japan).
Annexin V-FITC/PI stained fluorescence microscopy: Apoptosis assay was performed by using Annexin V-FITC/PI using previously described protocol with some modification [30]. For invitro fluorescent staining, 1x104 cells per well were seeded in 96 well-plate overnight. Cells were then treated with curcumin and TRAIL at various concentrations for 24 and 48 hour. Live cells were stained with FITC-annexin V (BD Biosciences, San Jose, CA) for 15 min in 1x biding buffer and then 5 μL of PI (Himedia) for 15 min at room temperature in the dark. Cells were washed three times with PBS and the fluorescent images were acquired using inverted fluorescence microscope with a camera (Nikon ECLIPSE Ti-S, Japan). Compartmental analysis bio-application module was used to quantify the fluorescence intensity of FITC-annexin V.
DNA content analysis: Cell cycle phase distribution with cellular DNA content was carried out using flow cytometry as described previously [31]. KCL22 cells were plated into 6-well plate at a density 1x106 cells/ml and treated with different concentrations of compounds for 24 hour. Cultured cells were then washed with cold PBS and fixed in 70% ethanol at -20°C for 2 hour. Fixed cells, treated with RNase A (10 mg/ml) and stained with propidium iodide (PI) are kept in dark for 30 min at room temperature. PI fluorescence of each nuclei was measured by flow cytometer (BD FACS Calibur, Becton Dickinson, USA). Data were analysed with the help of Cell Quest Pro V 3.2.1 software (Becton Dickinson, USA).
Statistical Analysis
Data of the cell viability were expressed as the mean±SEM from three independent experiments. One-way ANOVA and Dunnett’s Multiple Comparison Test was done using Graph Pad prism (Version 5.01) software for significance test, using a p-value≤0.05.
Results
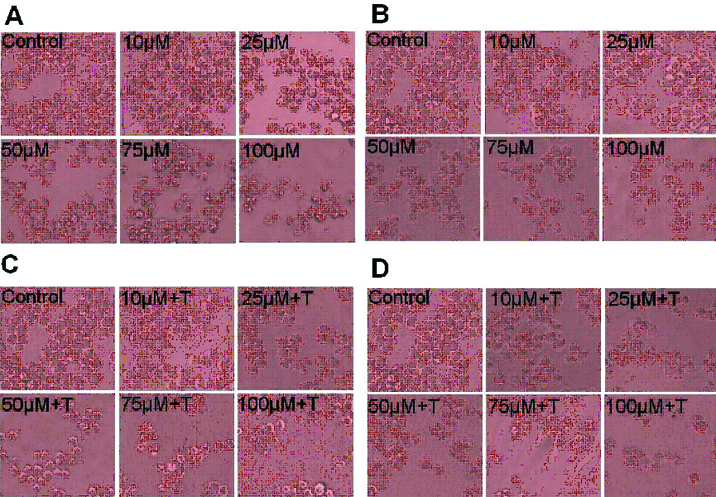
A. Effect of compounds on morphological changes and antiproliferative activity: Cellular morphological changes were observed in KCL22 cells at 24 and 48 hour after the treatment of curcumin (10, 25, 50, 75 and 100 μM) and TRAIL (10 nM) at various concentrations by inverted phase contrast microscopy. As shown in [Table/Fig-1], the unexposed control cells remained smooth and flat and also exhibited even cell surface, indicating normal healthy cells. However, there was drastic change in the morphology of the cells treated with curcumin and TRAIL as shown by the photomicrograph. Curcumin and TRAIL exposed cells displayed typical apoptotic feature; chromatin condensation, and extensive cytoplasmic vacuolization as compared to the unexposed control cells. Treated cells acquired round showing cellular shrinkage.
Morphological view of live and dead cells of KCL22 cell line (a) Cells treated with 10 to 100 μM of curcumin at 24 h (b) Cells treated with 10 to 100 μM of curcumin at 48 h. (c) Cells treated with 10 to 100 μM of curcumin plus TRAIL with at 24 h (d) Cells treated with 10 to 100 μM of curcumin plus TRAIL with at 48 h. Photomicrographs were taken by inverted phase contrast microscope (objective 20X).
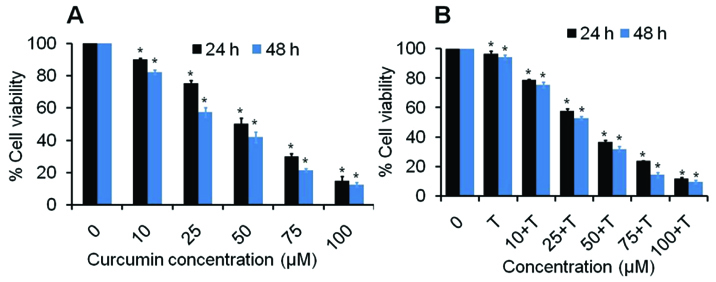
B. Combined Curcumin and TRAIL Treatment Induces Cytotoxicity in Myeloid Leukaemic Cells: The antiproliferative effects of different concentrations of curcumin against KCL22 cells were evaluated by MTT assay at 24 h and 48 h period [Table/Fig-2]. The cell viability data of KCL22 cells at 24 hour indicates that curcumin reduces the cell viability approximately 90.03, 75.47, 50.25, 29.91 and 14.81% (p<0.05) at 10, 25, 50, 75 and 100 μM of curcumin as compared to control. However, it was further reduced to approximately 82.26%, 57.34%,41.84%, 21.23 and 12.53% (p<0.05) due to 10, 25, 50, 75 and 100 μM doses of curcumin, respectively at 48 hour in a dose dependent manner. However, addition of TRAIL at 100 nM induced the cytotoxicity effects of curcumin. As a results, cells treated with combine dose of curcumin and TRAIL reduced the cells viability approximately 96.57, 78.72, 57.69, 36.77, 23.87, 11.73 and 94.14, 75.40, 53.00, 32.01, 14.37, 9.64% at 24 and 48 h, respectively both in dose and time dependent manner [Table/Fig-2]. These results suggested that the treatment of curcumin significantly reduce the cell viability of KCL22 cell line in dose and time dependent manner.
MTT assay of curcumin and TRAIL against KCL22 cells. (a) The percent cell viability of KCL22 cells treated with 10 to 100 μM of curcumin at 24 h and 48 h as described in the experimental section. (b) The percent cell viability of KCL22 cells treated with 10 to 100 μM of curcumin plus TRAIL at 24 h and 48 h. Values are expressed as means ± SEM of at least three independent experiments, *p<0.05 as compared with their respective control.
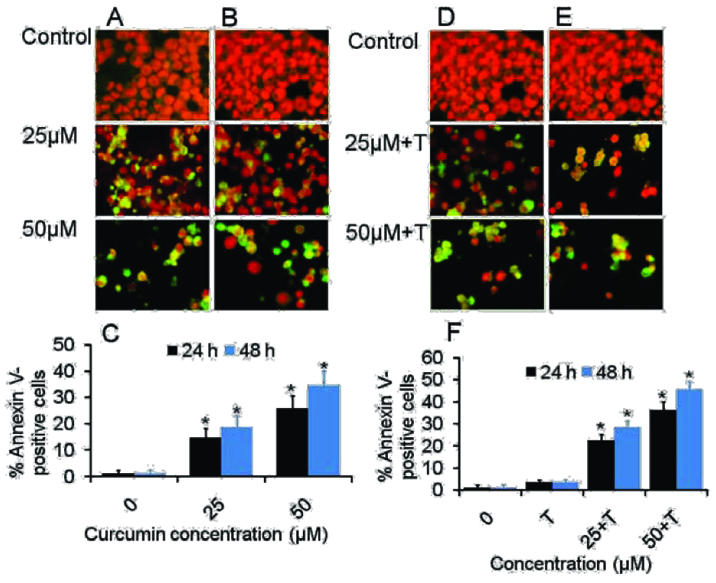
C. Annexin V-FITC/PI stained fluorescence microscopy: Since apoptotic cells with hypodiploid DNA content were detected in the sub-G1 phase of the cell cycle, another apoptosis marker, phosphatidylserine exposure was examined by Annexin V-FITC/PI assay using fluorescence imaging to further investigate if curcumin could induce apoptosis in the CML cancer cells. It was observed that curcumin induced apoptosis in KCL22 cells in dose- and time dependent manner [Table/Fig-3]. After 24 hour exposure to curcumin, increasing doses resulted in an increased proportion of apoptotic cells by approx two fold, from 14.66% in the 25μM curcumin treated cells to 26.00% in the 50 μM curcumin. Following 48 hour curcumin treatment, Annexin V-FITC-stained positive cells increased to 18.66% and 53% at 25 and 50 μM curcumin respectively. For curcumin and TRAIL treated cells, apoptotic cells were found to be most pronounced after treatment with the curcumin and TRAIL. The Annexin V-FITC-stained positive cells increased from 22.66 to 36.33% in 25 and 50 μM curcumin treated cells at 24 hour. However, these were dramatically increased from 28.66 to 45.66% at 48 h treatment [Table/Fig-4]. Interestingly, the apoptotic effect induced by TRAIL alone appeared to be less evident in KCL22 cells than curcumin treated cells.
Morphological assessment of Curcumin and TRAIL-induced apoptosis in KCL22 cells. (a&b) Representative figures of KCL22 cells were treated with curcumin for 24 h and 48 h respectively (d&e) Representative figures of KCL22 cells were treated with curcumin plus TRAIL for 24 h and 48 h respectively. Cells were stained with apoptosis marker annexin V (green) and nucleus marker PI (red). (c) Histogram shows mean % Annexin V positive cells in KCL22 cells treated with various concentration of curcumin for 24 h and 48 h. (c) Histogram shows mean % Annexin V positive cells in KCL22 cells treated with various concentration of curcumin for 24 h and 48 h. Data were shown in means±SEM of three independent experiments, *p<0.05 as compared with their respective control. All images were visualized and captured using inverted fluorescence microscope with a camera (Nikon ECLIPSE Ti-S, Japan) (objective 20X).
Effect of curcumin and TRAIL on different phases of cell cycle in KCL22 cells treated cells were stained with propidium iodide and measured by flow cytometry. Representative photomicrograph showing the apoptosis and phase distribution of cell population in (a) Control cells (b) TRAIL treated cells (c) cells treated with 50 μM of curcumin, (d) cells treated with 50 μM of curcumin and TRAIL.
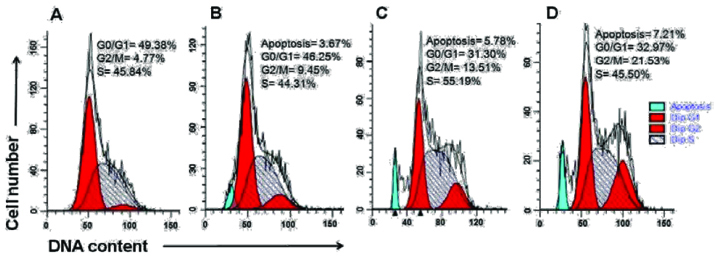
D.Curcumin modulates cellular DNA content and induces G2/M phase arrest: Flow cytometric cell cycle analysis was performed with cellular DNA content of KCL22 cells treated with 50 μM of curcumin and 100 nM TRAIL alone and in combination. Apoptotic cells were evaluated by figuring the number of sub-diploid cells in the cell cycle histogram. [Table/Fig-4] showing KCL22 cell exposed to 50 μM of curcumin alone and both curcumin and TRAIL, apoptotic cells were accounted to be 5.78 and 7.21% separately, when contrasted with control. However, at 100 nM of TRAIL treatment KCL22 cells, apoptotic cells were accounted to be marginally little to 3.67%. Analysis of cell cycle of KCL22 cells revealed that 50 μM of curcumin alone and both TRAIL and curcumin arrest the hypodiploid approximately 13.51% and 21.53% in G2/M phase, separately [Table/Fig-4c&d]. However, TRAIL arrests the cells slightly little approx 9.45% in G2/M phase indicating insignificant arrest of cells. This outcome demonstrated that curcumin and TRAIL altogehter induced G2/M phase arrest and lead to cell apoptosis.
Discussion
Cancer is the second leading cause of death in India [32]. Conventional therapies cause across the board systemic toxicity and lead to genuine reactions which deny their long haul use. Also, much of the time tumour resistance and its recurrence are generally watched. Consequently, there is a dire need to recognize suitable anticancer therapies that are very exact with negligible symptoms.
Recent studies have demonstrated that plant-derived dietary compounds provide protection against the development of cancers and other diseases [33,34]. Our results supports the previously reported data by other investigators that nourishment added substance curcumin restrains expansion and prompts apoptosis in a several tumour cell lines [35–39]. We have also demonstrated that curcumin sensitizes KCL-22 chronic myeloid leukaemia cells to TRAIL-induced apoptosis displayed typical apoptotic feature; chromatin condensation, and extensive cytoplasmic vacuolization [Table/Fig-1a-d].
Few reports have demonstrated the capacity of nontoxic concentrations of chemotherapeutic agents to sharpen tumour cells for apoptosis by death-inducing ligands, for example, FasL, TRAIL, or TNF-α [40–43].
Our study demonstrates that moderate concentration of TRAIL and curcumin alone do not induce appreciable cell death in KCL22 cells. Similarly KCL22 cell were also resistant to TRAIL at concentration ranging to 5 to 50ng/ml. In contrast, curcumin in combination with TRAIL enhance cell death in KCL22 cells at concentration that each agent alone is poorly effective. Collaboration in the middle of curcumin and TRAIL was additionally seen in prostate cancer cells [44,45].
Cytotoxicity of both curcumin and TRAIL treatment might occur because of TRAIL-induced apoptosis by curcumin. Treatment of KCL22 cells with curcumin and TRAIL together brought about a reduction in the diploid cells with a comparing increment in the hypodiploid (sub-G1) cells, recommending that cytotoxicity actuated by curcumin and TRAIL is intervened by incitement of apoptosis. In contrast, our study revealed that curcumin and TRAIL alone had little effect on apoptosis, together they induced significant apoptosis and curcumin enhances the apoptotic effect of TRAIL in dose and time dependent manner [Table/Fig-1a-d]. Our results supports the previously reported data by other investigators that anticancer agents such as doxorubicin, 5-fluorouracil, cisplatin, paclitaxel, etoposide, enhance TRAIL-mediated apoptosis in tumour cells at subtoxic concentrations [40,46,47].
The upgraded cytotoxicity of consolidated curcumin and TRAIL treatment might come about because TRAIL-induced apoptosis by curcumin. Indeed, treatment of KCL-22 cells with curcumin and TRAIL together bought a decrease in the diploid cells through arrest in hypodiploid cells in G2/M phase, suggesting that cytotoxicity induced by curcumin and TRAIL is mediated by induction of apoptosis [Table/Fig-4]. TRAIL arrests the cells slightly little approx 9.45% in G2/M phase showing negligible arrest of cells. These results indicated that curcumin alone induced G2/M phase arrest and lead to cell apoptosis more as compared to TRAIL alone. Whereas combination of both, curcumin enhances the TRAIL induced apoptosis via G2/M phase arrest [Table/Fig-4].
Since systemic toxicity of these agents is limited, curcumin, which is a pharmacologically safe agents, gives a better alternate to deal with sharpen tumour cells to TRAIL without toxicity. Resistance to apoptosis is a noteworthy deterrent in chemotherapeutic medications of cancer. The apoptotic property of curcumin, in conjunction with its non-harmful nature, could make it a conceivably powerful preventive and/or therapeutic agent against tumour. Besides, the blend of curcumin and TRAIL showed significant cytotoxicity in cancer cells, which presents general resistance to numerous other chemotherapeutic medicines. Along these lines, the combination of curcumin and TRAIL may be a novel regimen for the treatment of human malignancies that are resistant to chemotherapy. On the other hand, the potential clinical ramifications of our studies will rely upon regardless of whether curcumin can be offered securely to people at dosages sufficiently high to accomplish pharmacologically dynamic levels. Clinical trials of oral curcumin can’t be used as a result of poor bioavailability because of its fast digestion system in liver and intestinal divider. In this regard, invivo studies are expected to assess the efficacy of curcumin as a chemopreventive agent and/or helpful specialists for disease [48].
Conclusion
Our study concludes that curcumin inhibits cancer cell growth by inducing apoptosis and enhance the therapeutic potential of TRAIL which proposes that curcumin alone or in combination with TRAIL can be used for leukaemic cancer prevention and therapy. Curcumin could be a promising chemotherapeutic agent for human leukaemia. Along these lines, an additional invivo/invitro studies are needed to evaluate the efficacy of curcumin as a chemopreventive agent and combat the resistance of chemotherapeutic agent against cancer.
[1]. Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM, The biology of chronic myeloid leukaemiaN Engl J Med 1999 341:164-72. [Google Scholar]
[2]. Nowell PC, Hungerford DA, A minute chromosome in human chronic granulocytic leukaemiaScience 1960 132:1497 [Google Scholar]
[3]. Aggarwal BB, Kumar A, Bharti AC, Anticancer potential of curcumin: preclinical and clinical studiesAnticancer Res 2003 23:363-98. [Google Scholar]
[4]. Maheshwari RK, Singh AK, Gaddipati J, Srimal RC, Multiple biological activities of curcumin: a short reviewLife Sci 2006 78:2081-87. [Google Scholar]
[5]. Devasena T, Rajasekaran KN, Gunasekaran G, Viswanathan P, Menon VP, Anti Carcinogenic effect of bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione a curcumin analog on DMH-induced colon cancer modelPharmacol. Res 2003 47:133-40. [Google Scholar]
[6]. Lin YG, Kunnumakkara A, Curcumin Inhibits Tumour Growth and Angiogenesis in Ovarian Carcinoma by targeting the Nuclear Factor-B PathwayClin Cancer Res 2007 13:3423-30. [Google Scholar]
[7]. Guo Y, Shan Q, Gong Y, Curcumin induces apoptosis via simultaneously targeting AKT/mTOR and RAF/MEK/ERK survival signaling pathways in human leukaemia THP-1 cellsPharmazie 2014 69(3):229-33. [Google Scholar]
[8]. Aggarwal BB, Sung B, Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targetsTrends in Pharmacological Sciences 2009 30(2):85-94. [Google Scholar]
[9]. Kunnumakkara AB, Anand P, Aggarwal BB, Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteinsCancer Letters 2008 269(2):199-225. [Google Scholar]
[10]. Yu J, Peng Y, Wu LC, Curcumin down-regulates DNA methyltransferase 1 and plays an anti-leukaemic role in acute myeloid leukaemiaPLoS ONE 2013 8:2 [Google Scholar]
[11]. Aggarwal BB, Kumar A, Aggarwal MS, Shishodia S, In Phytochemicals in Cancer Chemoprevention; Preuss, H., Ed. 2005 New YorkCRC:349-87. [Google Scholar]
[12]. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB, Bioavailability of curcumin: problems and promisesMolecular Pharmaceutics 2007 4(6):807-18. [Google Scholar]
[13]. Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB, Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of I kappaB alpha kinase and Akt activationMol Pharmacol 2006 69:195-206. [Google Scholar]
[14]. Singh S, Khar A, Biological effects of curcumin and its role in cancer chemoprevention and therapyAnticancer Agents Med Chem 2006 6:259-70. [Google Scholar]
[15]. Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR, Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1Oncol Rep 2006 15:1557-62. [Google Scholar]
[16]. Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S, Effects of curcumin on tumour angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude miceClin Hemorheol Microcirc 2006 34:109-15. [Google Scholar]
[17]. Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Curcumin inhibits tumour growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathwayClin Cancer Res 2007 13:3423-30. [Google Scholar]
[18]. MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES, Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAILJ Biol Chem 1997 272:25417-20. [Google Scholar]
[19]. Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ, Intracellular regulation of TRAIL-induced apoptosis in human melanoma cellsJ Immunol 1998 161:2833-40. [Google Scholar]
[20]. Suliman A, Lam A, Datta R, Srivastava RK, Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathwaysOncogene 2001 20(17):2122-33. [Google Scholar]
[21]. Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A, Induction of apoptosis by Apo-2 ligand, a new member of the tumour necrosis factor cytokine familyJ Biol Chem 1996 271:12687-90. [Google Scholar]
[22]. Zang DY, Goodwin RG, Loken MR, Bryant E, Deeg HJ, Expression of tumour necrosis factor-related apoptosis-inducing ligand, Apo2L, and its receptors in myelodysplastic syndrome: effects on invitro hemopoiesisBlood 2001 98:3058-65. [Google Scholar]
[23]. Plasilova M, Zivny J, Jelinek J, Neuwirtova R, Cermak J, Necas E, TRAIL (Apo2L) suppresses growth of primary human leukaemia and myelodysplasia progenitorsLeukaemia 2002 16:67-73. [Google Scholar]
[24]. De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1Nature 1999 401:489-93. [Google Scholar]
[25]. Zamai L, Secchiero P, Pierpaoli S, Bassini A, Papa S, Alnemri ES, TNF-related apoptosis-inducing ligand (TRAIL) as a negative regulator of normal human erythropoiesisBlood 2000 95:3716-24. [Google Scholar]
[26]. Secchiero P, Melloni E, Heikinheimo M, Mannisto S, Di Pietro R, Iacone A, Zauli G, TRAIL regulates normal erythroid maturation through an ERK-dependent pathwayBlood 2004 103:517-522. [Google Scholar]
[27]. Schmidt U, van den Akker E, Parren-van Amelsvoort M, Litos G, De Bruijn M, Gutierrez L, Btk is required for an efficient response to erythropoietin and for SCF-controlled protection against TRAIL in erythroid cellsJ Exp Med 2004 199:785-95. [Google Scholar]
[28]. Campioni D, Secchiero P, Corallini F, Melloni E, Capitani S, Lanza F, Evidence for a Role of TNF-Related Apoptosis-Inducing Ligand (TRAIL) in the Anemia of Myelodysplastic SyndromesAm J Pathol 2005 166(2):557-63. [Google Scholar]
[29]. Ashfaq M, Singh S, Sharma A, Verma N, Cytotoxic evaluation of the hierarchal web of carbon micro-nanofibersInd Eng Chem Res 2013 52:4672-82. [Google Scholar]
[30]. Looi CY, Arya A, Cheah FK, Muharram B, Leong KH, Mohamad K, Induction of apoptosis in human breast cancer cells via caspase pathway by vernodalin isolated from Centratherum anthelminticum (L.) seedsPLoS One 2013 8(2):e56643 [Google Scholar]
[31]. Ahamad MS, Siddiqui S, Jafri A, Ahmad S, Afzal M, Arshad M, Induction of Apoptosis and Antiproliferative Activity of Naringenin in Human Epidermoid Carcinoma Cell through ROS Generation and Cell Cycle ArrestPLoS One 2014 9(10):e110003 [Google Scholar]
[32]. Ali I, Wani WA, Saleem K, Cancer Scenario in India with Future PerspectivesCancer Therapy 2011 8:56-70. [Google Scholar]
[33]. Block G, Patterson B, Subar A, Fruit, vegetables, and cancer prevention: A review of the epidemiological evidenceNutr Cancer 1992 18:1-29. [Google Scholar]
[34]. Messina M, Barnes S, The role of soy products in reducing the risk of cancerJ Natl Cancer Inst 1991 83:541-46. [Google Scholar]
[35]. Chen HW, Huang HC, Effect of curcumin on cell cycle progression and apoptosis in vascular smooth cellsBr J Pharmacol 1998 124:1029-40. [Google Scholar]
[36]. Mukhopadyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB, Curcumin-induced suppression of cell proliferation correlates with downregulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylationOncogene 2002 21:8852-61. [Google Scholar]
[37]. Anto RJ, Mukhopadadhyay A, Denning K, Aggarwal BB, Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xLCarcinogenesis 2002 23:143-50. [Google Scholar]
[38]. Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal B, Curcumin downregulates cell survival mechanisms in human prostate cancer cell linesOncogene 2001 20:597-609. [Google Scholar]
[39]. Gautam SC, Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Nonselective inhibition of proliferation of transformed and nontransformed cells by the anticancer agent curcumin (diferuloylmethane)Biochem Pharmacol 1998 55:1333-37. [Google Scholar]
[40]. Nimmanapalli R, Perkins CL, Orlando M, O’Bryan E, Nguyen D, Bhalla KN, Pretreatment with paclitaxel enhances Apo-2 ligand/tumour necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levelsCancer Res 2001 61:759-63. [Google Scholar]
[41]. Borner MM, Myers CE, Sartor O, Sei Y, Toko T, Trepel JB, Drug-induced apoptosis is not necessarily dependent on macromolecular synthesis or proliferation in the p53-negative human prostate cancer cell line PC-3Cancer Res 1995 53:2122-28. [Google Scholar]
[42]. Sherwood ER, Ford TR, Lee C, Kozlowski JM, Therapeutic efficacy of recombinant tumour necrosis factor α in an experimental model of human prostatic carcinomaJ Biol Response Modif 1998 9:44-52. [Google Scholar]
[43]. Nakajima Y, DelliPizzi A, Mallouh C, Ferreri NR, Effect of tumour necrosis factor α and interferon α on the growth of human prostate cancer cell linesUrol Res 1995 23:205-10. [Google Scholar]
[44]. Deeb D, Jiang H, Gao X, Hafner MS, Wong H, Divine G, Curcumin sensitizes prostate cancer cells to tumour necrosis factor–related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-KB through suppression of IKBA phosphorylationMol Cancer Ther 2004 3(7):803-12. [Google Scholar]
[45]. Deeb D, Xu YX, Jiang H, Curcumin (diferuloyl-methane) enhances tumour necrosis factor–related apoptosis-inducing ligand-induced apoptosis in LNCaP prostate cancer cellsMol Cancer Ther 2003 2:95-103. [Google Scholar]
[46]. Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S, Chemotherapy augments TRAIL-induced apoptosis in breast cell linesCancer Res 1999 59:734-41. [Google Scholar]
[47]. Sun SY, Yue P, Hong WK, Lotan R, Augmentation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by the synthetic retinoid 6[3-(1-adamantyl)-4-hydroxyphenyl]-2 napthalene carboxylic acid (CD437) through up-regulation of TRAIL receptors in human lung cancer cellsCancer Res 2000 60:7149-55. [Google Scholar]
[48]. Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancerClin Cancer Res 2001 7:1894-900. [Google Scholar]