In search of pleasure and contentment, humans seek the aid of various drugs, amongst which tobacco tops the list. It is abused in two forms i.e. smoking and smokeless tobacco [1]. Globally smoking is the dominant form of tobacco use as cigarette is readily available and is a cheaper form of tobacco. India is a second largest consumer of tobacco products and third largest producer of tobacco in the world. In present scenario, tobacco continues to be the leading cause of preventable death, resulting in an expected increase from 1.5 to 7 million annually by 2020 around the globe [2].
Cigarette smoke posses a significant human health hazard, especially affecting vascular hemodynamic and multisystem involvement. Cigarette smoking exacerbates reactive oxygen species (ROS) including superoxide (O2-), hydrogen peroxide (H2O2-), peroxyl radical (ROO-) and hydroxyl radicals (OH-) formation. Antioxidants protect body cells from damaging effect of oxidation. In normal healthy individuals, the free radicals formed, are quenched and removed by antioxidant defense mechanisms. On the contrary, in tobacco users, the removal of free radicals is disturbed because of depletion of antioxidant nutrients by tobacco by-products and results in oxidative stress [3]. The term oxidative stress is used to describe the imbalance between oxidants & antioxidants. Numerous oxidants present in tobacco, especially consumed through smoking, damages the vital bio-molecules at the cellular level like DNA, RNA, lipids, amino acids, dietary and endogenously produced antioxidants such as Glutathione [4]. However, the effect of these free radicals on α-Lipoic acid is not well documented in the literature.
The role of oxidative stress in diseases associated with endogenous free radical injury is well documented whereas the effect of exogenous substances like tobacco and its correlation with antioxidant levels need further exploration. It has been seen that tobacco depletes the body vitamin levels which protect us from oxidative stress, a causative agent of various diseases [5].
Various biomarkers have been identified till date for the assessment of oxidative stress in vivo for early diagnosis and safe prognosis due to efficient use of drugs, supplements and dietary nutrition. S. Malondialdehyde (S.MDA) is an indicator of increased lipid peroxidation caused by free radical toxicity. There is a need to identify the levels of antioxidants required to combat this increasing levels of toxic radicals.
The present study was designed with an aim to find out the level and its correlation of the antioxidants produced endogenously like S. Glutathione (S.GSH) and S. α- Lipoic Acid (S. α-LA), during increased load of MDA as a result of tobacco consumption.
Materials and Methods
The present study was carried out in the Department of Physiology of a tertiary care hospital. This cross-sectional, case control study included 200 participants, chosen randomly from contract labor in the institutional campus and not occupationally exposed to metals and pesticides. The controls were non tobacco users, healthy volunteers, free from any chronic substance abuse and systemic disease, recruited from normal population visiting our hospital, as relatives of patients and medical students and faculty during August 2013 to December 2014.
On the basis of pilot study done in our hospital and study conducted on prevalence in Udaipur city [6], we calculated the incidence of tobacco consumer to be 66%. We also found out the percentage of healthy controls, meeting all the inclusion and exclusion criteria, to be around 22%. The sample obtained, was proportionally divided into cases and controls in the ratio of approximately 3:1.
All the age matched (18-50 years), sex matched (149 male & 41 female) participants were broadly divided into two groups (A & B); group A comprised of tobacco users (n=150) with history of smoking cigarette/biddies and chewing tobacco daily, for at least one year and group B had controls non tobacco users (n=50). Smoking index parameters used for the quantification of cumulative smoking exposure [7] and for grading of chewing is done [8]. The subjects with any systemic disease, obesity, severe anaemia, alcoholics and past tobacco users were excluded from the study. Further, it was ensured that none of the subjects from either group were on nutritional supplementation or any drug altering the level of antioxidants. The study was approved by ethical committee of Geetanjali University, Udaipur on 15/05/2013.
After obtaining the ethical clearance from institutional ethics committee, the data from both the groups were collected in the detailed proforma along with requisite physical examination. For the, biochemical analysis, 5 ml of blood was drawn after an overnight fast (12 h) by venous puncture from both the groups. After clotting of the blood sample, serum was separated by a centrifugation at 3000 rpm for 10 minutes and used for biochemical analysis for S. MDA, S. GSH and S. α-LA.
S.MDA was estimated by thiobarbituric acid (TBA) assay method [9]. Spectro-photometric method was used for the determination of S. α-LA by using MBTH (3-Methyl-2-Benzothiazolinone hydrazone hydrochloride) and ferric chloride [10] and S. GSH was estimated by Beutler’s improved method [11].
Statistical Analysis
The data was analysed by using Statistical Package for the Social Sciences (SPSS) for Windows Version 16.0 (SPSS). Comparison of mean of continuous data between group-A and group-B were tested by unpaired Student t-test. A p-value of <0.01 was used to establish statistical significance. Correlation between the antioxidants and S. MDA was analysed using Pearson’s correlation (r) coefficient test.
Results
Lipid peroxidation products (S. MDA) level was significantly higher (p<0.0001) in tobacco users as compared to non tobacco users (2.72 ± 0.87 nmol/ml and 1.39 ± 0.47 nmol/ml, respectively; [Table/Fig-1,2]). The levels of S.GSH and S. α- LA were significantly lower (p<0.0001) in tobacco users as compared to non tobacco users [Table/Fig-1,3,4].
S. MDA, S. GSH and S. α - LA levels in Tobacco & Non Tobacco users. (*p<0.0001)
| Variables | Group-A | Group-B | t- value | p- value |
|---|
| Tobacco users | Non Tobacco users |
|---|
| S.MDA (nmol/ml) | 2.72 ± 0.87 | 1.39 ± 0.47 | 13.67* | <0.0001 |
| S.GSH (mg/dl) | 23.24 ± 7.42 | 32.82 ± 2.95 | 13.02* | <0.0001 |
| S. α - LA (μg/ml) | 9.94 ± 5.96 | 14.24 ± 4.34 | 5.49* | <0.0001 |
Comparison of S.MDA level (nmol/ml) in tobacco and nontobacco users.
*p<0.0001
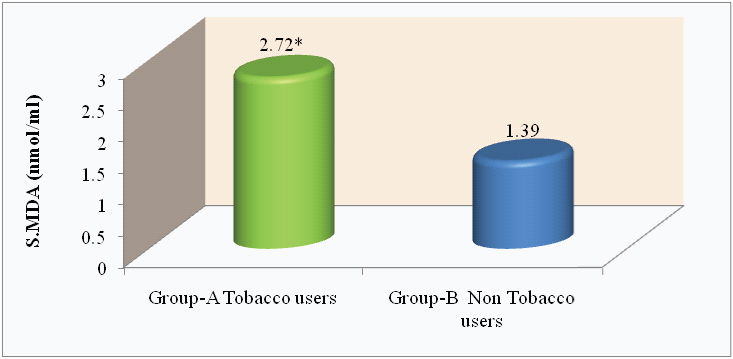
Comparison of S.GSH level (mg/dl) in tobacco and nontobacco users.
*p<0.0001
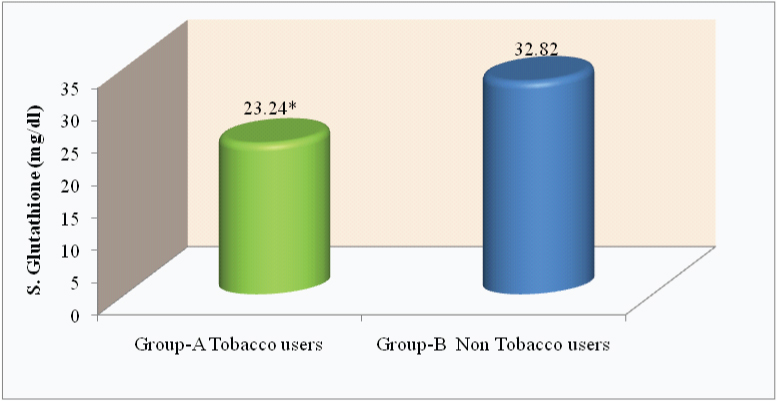
Comparison of S. α - LA level (μg/ml) in tobacco and nontobacco users.
*p<0.0001
In the present study, the level of α - LA was within normal range in both the groups. However, highly significant (p<0.0001) reduction in the levels were seen in tobacco users (group -A) as compared to non tobacco uses (group- B).
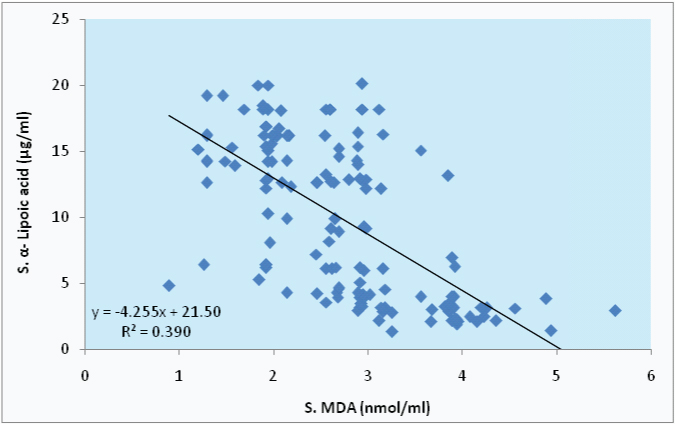
In the present study, S.MDA level shows highly significant and strong negative correlation with S.GSH and S. α- LA among tobacco users, with r=-0.619, r=-0.625 respectively; as seen in [Table/Fig-5,6 and 7].
Correlation between S. MDA and antioxidants (S. GSH & S. α-LA) in group A & group B. (*p<0.0001)
| Variable | Group-A Tobacco users | Group-B Non Tobacco users |
|---|
| r-value | p-value | r-value | p-value |
|---|
| S. GSH | -0.619* | <0.01 | 0.193 | <0.05 |
| S. α- LA | -0.625* | <0.01 | 0.500 | <0.01 |
Correlation between S. MDA and S.GSH in tobacco users.
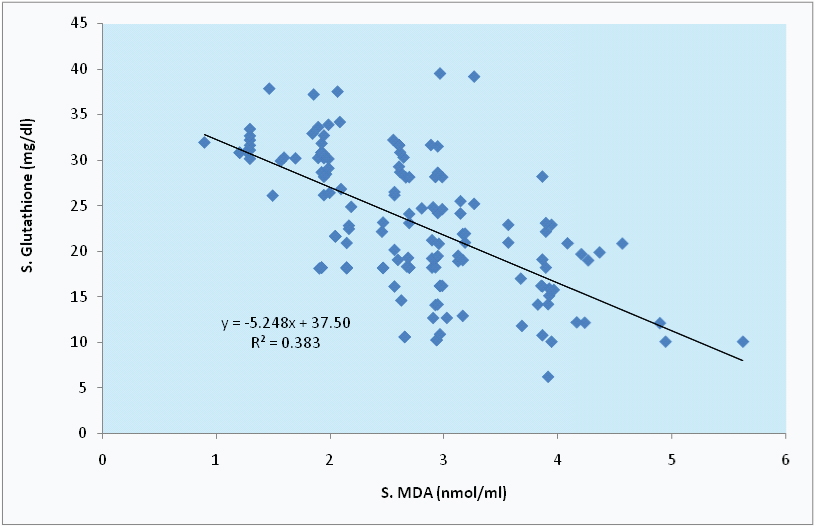
Correlation between S. MDA and S. α- LA in tobacco users.
Discussion
The present study has documented a significant increase (p<0.0001) in S.MDA levels in tobacco consumers as compared to nontobacco users. Increase S.MDA is due to excessive formation of reactive oxygen species (ROS) which represents oxidative stress, caused by exogenous sources like tobacco and leads to peroxidation of lipids [12]. S.MDA levels could be a reflection of cellular injury caused by lipid peroxidation; which is a mechanism of oxidative breakdown of the lipid molecules resulting in accumulation of large amounts of toxic by products. These metabolites in turn damages membrane structures directly and distant targets indirectly through the evolution of toxicity by reactive alkanals [13].
Shorter life span pose difficulty in direct measurement of free radicals, hence their estimation could be done indirectly by measuring the end products of the lipid peroxidation, which could give a fair reflection of the free radical status of an individual. Therefore, the extent of lipid peroxidation and free radical mediated damage are assayed by S.MDA levels. Though the literature doesn’t provide any information regarding the critical S.MDA levels, indicative of severity of tissue damage, but they have a direct and strong association with peroxidation of polyunsaturated fatty acid (PUFA) showing the extent of oxidative damage [14].
Tobacco by-products, form many hazardous compounds to react with biological macromolecules, leading to rise in oxidative stress and formation of ROS. These free radicals have cytotoxic sequences which result in peroxidation of membrane phospholipids and alter membrane liquidity, increase permeability and loss of integrity of cellular membranes [15,16].
As the preventive shield against this excessive generation of ROS, the body is equipped with the oxidant scavenging system, which includes preventive antioxidants viz. GSH, reducing the initiation of chain of reactions involved in lipid peroxidation while the other type blocks the progression of the already initiated process of lipid peroxidation, viz. S. α-LA.
Apart from free radical excess, the present study also reports a significant fall (p<0.0001) in the levels of S.GSH and S. α-LA in tobacco users (group-A) as compared to non tobacco users (group-B).
This decrease in the levels of S. GSH in smokers is well documented, as the toxic chemicals present in tobacco disturbs the normal glutathione cycle in red blood cells in which the reduced S. GSH, an important sensitive antioxidant protecting the RBC. The tripeptide Glutathione (gamma-L-glutamyl-Lcysteinylglycine), is the major intracellular non-protein thiol compound playing a major role in the protection of cells and tissue structures [17]. It acts as a nucleophilic scavenger and an antioxidant in electrophilic/oxidative tissue injuries resulting from free radical excess.
S. α- LA (8-thioctic acid) is another thiol with antioxidant properties, involved in recycling of vitamin C and/ or vitamin E thus increasing their availability to cells. The α-LA, acts as a direct scavenger of ROS but its effects are more pronounced by modulation of the endogenous GSH levels [18]. It induces the Nrf2- mediated gene expression regulating GSH synthesis and increasing the substrate availability for the same [19]. Apart from this, S. α- LA has a strong anti-apoptotic action by limiting TNF- α induced NF-κB activation and adhesion molecule expression [20,21]. It also appears to exert its anti apoptotic effect by activating a protein kinase B/Akt, mediating cell survival by regulating the phosphorylation state of stress response enzyme in insulin signaling pathway [22]. Most of the studies on the role of α-LA have been conducted on in- vitro/ cell culture models and the exact mechanism of action, in human subjects, still remains to be explored. The present study, however, has tried to establish the causal relationship of decreased levels of α-LA in tobacco consumers, rendering them vulnerable to oxidative tissue damage owing to accumulation of toxic free radicals.
Further, our study has established a strong negative and highly significant (p<0.01) correlation of S.MDA level with S.GSH and S. α-LA (r= -0.619, r= -0.625 respectively) among tobacco users. This strong negative correlation of antioxidants with S.MDA excess is suggestive of accumulation of toxic free radicals and resultant depletion of S. GSH and S. α- LA. It becomes evident that smokers are more prone of oxidative tissue damage, apoptosis, abnormal gene expression and tobacco related morbidity, which could result in abnormal cellular function.
Limitations
Though the study has been designed and conducted carefully taking care of all the stringent precautions but there is a scope of improvement in this study by the biochemical estimation of tobacco by-products/metabolites. Further, the various biochemical assessments had been done using the manual methods; hence the use of available kits could have yielded more sensitivity to the results, though the specificity might be same in both the methods.
Conclusion
To conclude, the decreased levels of α-Lipoic acid and GSH, in our study, clearly indicates the potential risk of cellular damage in tobacco users due to lipid peroxidation. As we know, antioxidant protection is never 100% efficient, thus mechanism of repair is one of the key for survival. Hence, the recommendation to supplement S. α-Lipoic acid and Vitamin C, in tobacco users can prevent this damage where as quitting this evil habit will be the best available option.