Introduction
Infectious Granulomatous Dermatoses (IGDS) have various aetiological factors with a considerable overlap in the histopathological and clinical features, thus posing a diagnostic dilemma for dermatologists and pathologists.
Aim
We aimed at determining the histopathological profile of IGDS correlating it with clinical features with an attempt to find the aetiology.
Materials and Methods
In a cross-sectional study conducted in a tertiary referral center of Mumbai over two years, out of 1872 skin biopsies received, 239 histopathologically diagnosed cases of IGDS were studied for histopathological features of granuloma. A clinico-histopathological correlation was attempted. Chi-square test was used for comparison of proportions of different groups.
Results
Leprosy (211 cases) and tuberculosis (28 cases) were the commonest histopathologically diagnosed IGDS. Leprosy spectrum included BT (30.33% cases), followed by TT (21.32%), BL and LL and 21.79% cases of lepra reactions. Skin TB biopsies on histopathology showed lupus vulgaris (53.85% cases), scrofuloderma (15.38%), TBVC and papulonecrotic tuberculid (11.54% each). In leprosy maximum clinico-pathological agreement was seen at tuberculoid pole (TT 72.7% and BT 56.6%). Among tuberculosis cases, scrofuloderma (100%) and lupus vulgaris (53.8%) showed maximum agreement.
Conclusion
Leprosy and skin TB are the commonest IGDS in Mumbai region though difficult to diagnose and subcategorize with certainty during initial stages. Histopathology plays the important role to elucidate the dilemma. This being a single center study, more such studies with a larger sample size are recommended to get more elaborate data and regional prevalence of these IGDS for a better overall approach to prevention, treatment and control.
Introduction
Infectious Granulomatous Dermatoses (IGDS) are a large family having various aetiologies but sharing the common histological denominator of granuloma formation. Thus similar histological pattern can be produced by several causes, and, conversely, a single cause may produce varied histological patterns thus posing a challenge to clinicians and dermatopathologists. During initial stages, it is difficult to arrive at a diagnosis only on clinical basis. Thus clinico-histological correlation plays a pivotal role in such circumstances. Many previous studies have reported a high prevalence of IGDS in the developing countries with a marked variation in their frequency across different regions. Several authors including Amanjit Bal [1] from Punjab, North India, S Dhar [2] from Kolkata, Eastern India and Keyoor Gautam [3] from South India have reported 87.8%, 83.36% and 65.5% cases respectively of IGDS out of granulomatous dermatoses studied. Kaur et al., reported 0.02% incidence of cutaneous TB in Amritsar, North India [4]. Similarly Zafar et al., from Pakistan studied 2862 biopsies and found 4.29% cases of granulomatous dermatoses with 3.69% cases of cutaneous tuberculosis [5,6]. We conducted a cross-sectional study on IGDS in our government funded tertiary referral institute in Mumbai region of Maharashtra As to our knowledge, this was the first study of its kind conducted in western Maharashtra, targeting poor and middle class socio-economic strata patients attending the skin OPD.
Aim
We aimed at describing the clinical and histopathological profile of IGDS and differentiate them on the basis of granulomas and their variable histopathology and establish a clinical correlation.
Materials And Methods
In the present prospective study, a total of 1872 skin biopsies submitted in the Department of Pathology in Grant Medical College and Sir. JJ Group of hospitals for a period of two years between July 2009 to June 2011 was considered. All new cases and follow up cases which were clinically suspected and histopathologically diagnosed as IGDS were included in the study. Skin biopsies with a clinical suspicion of IGDS but found inadequate for histopathological processing were excluded. The detailed clinical history including age, sex, duration of disease and treatment received were taken into account. The cases were studied for histopathological features of granuloma, predominant cell, location of granuloma in the dermis and epidermal changes if any. All these data were carefully tabulated and a clinico-histopathological correlation was attempted. Skin biopsies were fixed, processed, stained with H & E, Modified Fite–Faraco stain, ZN stain, PAS stain, GMS stain, Reticulin stain, Giemsa stain and studied. Percentages were calculated for categorical variables. Chi-square test was used for comparison of proportions of different groups. All p-values < 0.05 were considered significant, while p-values between 0.05 and 0.10 were considered marginally significant.
Results
Out of the total 1872 skin biopsies received in our department in two year period, 273 (14.58%) cases were clinically diagnosed as granulomatous dermatoses. Out of 273, 207(75.82%) biopsies were sent with a clinical suspicion of various forms of leprosy. Cutaneous tuberculosis was the second most common clinical diagnosis {29/273 (10.62%)}. In rest 37 cases, non-infectious granulomatous lesions were clinically suspected. Out of 273 biopsies, ten were excluded from the study and 239 cases were histopathologically confirmed as IGDS. Out of 239 cases of IGDS, the most commonly diagnosed lesion after histopathological examination was leprosy (n=211) (77.28%) followed by cutaneous tuberculosis (28 cases). Out of the typified 211 cases of leprosy, borderline tuberculoid leprosy (BT) [Table/Fig-1] constituted the most common diagnosis followed by tuberculoid leprosy (TT) [Table/Fig-2], borderline lepromatous (BL) [Table/Fig-3] and lepromatous leprosy (LL) [Table/Fig-3], the distribution of which is shown in [Table/Fig-4]. Reactions constituted 21.79% of the cases out of which type I reactions were 10.42% and ENL [Table/Fig-5] were diagnosed in 11.37% cases. The age distribution pattern indicated that maximum (84.36%) leprosy cases were found between age group of 11-50 years with the highest percentage in the 21-30 year age group (36.17%). The second most commonly affected age group was 31-40 years (total 41 patients- 19.80%). However, the agewise distribution of paucibacillary and multibacillary lesions was found to be statistically insignificant (p-value=0.085) [Table/Fig-6]. The sex distribution pattern of leprosy revealed a male preponderance of 79.42% compared to 20.58 % females. Sexwise distribution of paucibacillary lesions, multibacillary leions and lepra reactions showed a marginal statistical significance with a p-value of 0.0519 [Table/Fig-7]. Upper extremity (29% cases) was the most common site involved followed by lower extremity (23% cases) and face (18% cases).
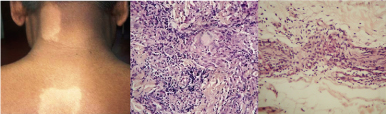
(a) Clinical photograph of a borderline tuberculoid patient showing hypopigmented patches with satellite lesions. (b) Histopathology of BT showing periadnexal epithelioid cell granuloma with scattered lymphocytes and Langhans giant cells. (H&E, X200) (c) Histopathology of BT showing intraneural epithelioid cell granuloma with lymphocytes infiltrating the nerve. (H&E, X200)
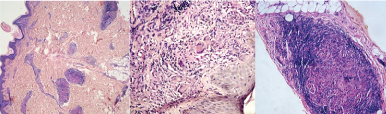
(a) Histopathology of TT showing well formed granulomas perivascular, perineural and periadnexal in location. (H&E, X40) (b) Histopathology of TT showing multiple epithelioid cell granulomas with Langhans giant cells and epidermal involvement (H&E, X200). (c) Histopathology of TT showing epithelioid cell granuloma infiltrating and damaging the nerve. Dense lymphocytic cuff can be seen (H&E, X200).
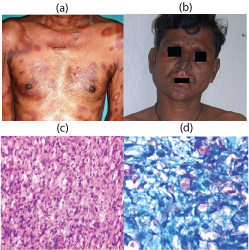
(a) Clinical photograph of Borderline leprosy with annular saucer shaped lesions of varying sizes showing central depression (b) Clinical photograph of patient with lepromatous leprosy showing leonine facies with nodules and plaques on the rim of ears, upper lip and nose and loss of eyebrows (c) Histopathology of LL showing sheets of foamy macrophages with very few lymphocytes. (H&E, X400) (d) Histopathology of lepromatous leprosy showing globi containing fragmented lepra bacill. (Fite Farraco, X1000)
Distribution of histopathologically diagnosed cases of leprosy.
| Sr. No. | Histopathological Diagnosis | No. of Cases(n=211) | Percentage |
|---|
| 1 | TT | 45 | 21.33% |
| 2 | BT | 64 | 30.33% |
| 3 | BB | 4 | 1.90% |
| 4 | BL | 25 | 11.85% |
| 5 | LL | 23 | 10.90% |
| 6 | Histioid Hansen | 3 | 1.42% |
| 7 | Borderline Type 1 | 22 | 10.42% |
| 8 | ENL | 24 | 11.37% |
| 9 | Neuritic Hansen | 1 | 0.47% |
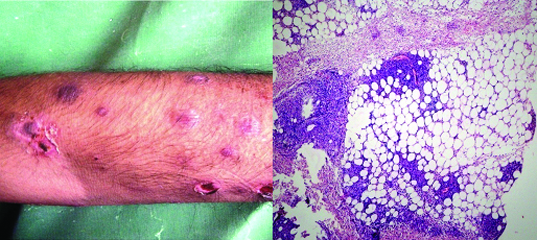
(a) Clinical picture of bullous ENL showing leprosy lesions with erythema, bulla formation and ulceration (b) Histopathology of ENL showing foamy macrophage granuloma with dense neutrophilic infiltrate which is also involving the subcutaneous adipose tissue. (H&E, X100).
Age wise statistical analysis of paucibacillary and multibacillary lesions. (*At Degree of Freedom=1; which is calculated by formula= (no. of rows-1)(no. of columns-1))
| Type of Lesion | Age | Total | Chi-square | p-value |
|---|
| <20 Years | >20 Years |
|---|
| Pauci-Bacillary | 24 | 84 | 108 | 2.958* | 0.085 |
| Multi-Bacillary | 5 | 47 | 52 |
| Total | 29 | 131 | 160 |
Sex wise statistical analysis of paucibacillary and multibacillary lesions. (*At Degree of Freedom=2; which is calculated by formula= (no. of rows-1)(no. of columns-1)
| Type of Lesion | Age | Total | Chi-square | p-value |
|---|
| Male | Female |
|---|
| Pauci-Bacillary | 86 | 24 | 110 | 5.94* | 0.0519 |
| Multi-Bacillary | 49 | 6 | 55 |
| Leprosy Reactions | 32 | 14 | 46 |
| Total | 187 | 44 | 211 |
Out of 26 histopathologically proven cases of cutaneous TB, lupus vulgaris (14 biopsies) [Table/Fig-8] was the most common histopathological diagnosis (53.85%). The second most common was scrofuloderma (4 cases). TBVC [Table/Fig-9] and papulonecrotictuberculid [Table/Fig-10] were diagnosed in three biopsies each. The most commonly affected age group in our study was 21-30 years with five out of 14 cases of lupus vulgaris, two out of four cases of scrofuloderma and two out of three cases of TBVC seen in this age group. In our study, lupus vulgaris was found equally in both sexes (seven cases each). Scrofuloderma, TBVC and papulonecrotic tuberculid showed male preponderance. Most commonly affected was lower extremity. TBVC and LV were more common on lower limbs. Scrofuloderma affected both upper and lower extremity. Out of 17 clinically suspected cases of lupus vulgaris, histopathological agreement was found in 10 biopsies (58.38%). Scrofuloderma cases showed 100% clinico-pathological correlation. While TBVC and papulonecrotictuberculid showed a clinico-patholopgical correlation in 50% and 25% cases respectively [Table/Fig-11].
Distribution of histopathologically diagnosed cases of cutaneous tuberculosis.
| S. No. | Histopathological diagnosis | No. of Cases | Percentage |
|---|
| 1 | Lupus Vulgaris | 14 | 53.85% |
| 2 | Scrofuloderma | 4 | 15.38% |
| 3 | TBVC | 3 | 11.54% |
| 4 | Cutaneous TB | 2 | 7.69% |
| 5 | PapulonecroticTuberculid | 3 | 11.54% |
| | 26 | 100.00% |
(a) Lupus vulgaris-Clinical photograph showing a plaque with a slightly elevated verrucose border and central atrophy (b) Histopathology of Lupus vulgaris showing acanthotic epidermis and superficial dermis showing well formed epitheloid cell granulomas, dense lymphocytic infiltrate. (H&E, X100).
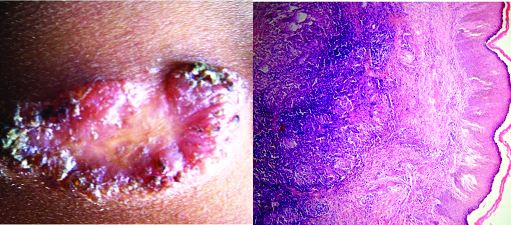
(a) TBVC- Clinical photograph showing verrucous ulcerative lesion involving sole of foot (b) Histopathology of TBVC showing acanthotic epidermis with superficial dermis infiltrated by dense PMN infiltrate. (H&E, X200).
Correlation between clinical and histopathological diagnosis in various forms of cutaneous tuberculosis.
| Sr. No. | Clinical Diagnosis | HISTOPATHOLOGICAL DIAGNOSIS | No. of Cases |
|---|
| LV | SCF | TBVC | Cut TB | PNT | GA | Sarcoidosis |
|---|
| 1 | LV | 10(58.83%) | - | 2(11.76%) | 2(11.76%) | 1(5.88%) | 1(5.88%) | 1(5.88%) | 17 |
| 2 | SCF | - | 3(100%) | - | - | - | - | - | 3 |
| 3 | TBVC | 1(50%) | - | 1(50%) | - | - | - | - | 2 |
| 4 | Cut TB | 1(33.3%) | 1(33.3%) | - | - | 1(33.3%) | - | - | 3 |
| 5 | PNT | 2(50%) | - | - | - | 1(25%) | - | 1(25%) | 4 |
| | 14(48.27%) | 4(13.79%) | 3(10.34%) | 2(6.89%) | 3(10.34%) | 1(3.44%) | 2(6.89%) | 29 |
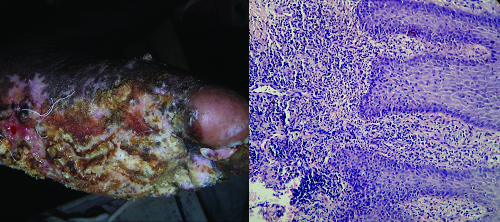
Two cases of granulomatous reaction to fungus were found on histopathology and diagnosis of chromoblastomycosis and blastomycosis [Table/Fig-12] were given.
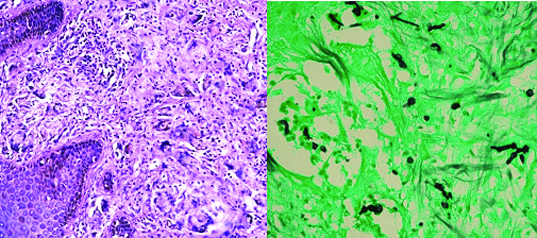
(a) Histopathology of chromoblastomycosis showing superficial dermis with granulomas and numerous foreign body giant cells. (H&E, X100) (b) Histopathology of chromoblastomycosis with thick walled fungal spores in chains in dermis. (GMS, X400).
Discussion
The commonest aetiology of IGDS in our study was leprosy (211 cases, 88.28%) followed by cutaneous TB (28 cases, 11.71%). In a similar study by Amanjit Bal [1], out of a total 515 biopsies of IGDS, 72.4% cases were diagnosed to have leprosy followed by 23.1% cases of cutaneous tuberculosis. Gautam K et al., reported similar results [3]. Out of the typified 211 cases of leprosy, borderline tuberculoid leprosy constituted the most common diagnosis (30.33%) followed by tuberculoid leprosy (21.33%). We can conclude that the leprosy subtypes BT and TT of paucibacillary leprosy are found to be more prevalent in this population of Mumbai. Similar findings have been reported by Amanjit Bal from Punjab, Keyoor Gautam from Nepal, Usha Manandhar from Manglore, and, Amrish Pandya from Gujrat, who found BT to be the commonest histological subtype {55%, 22%, 40% and 47.6% cases respectively} [1,3,7,8]. While Chauhary and Mehta from Gujrat and Jindal N et al., from Himanchal Pradesh found maximum cases at lepromatous pole (31.8% and 33.12% respectively) [9,10].
In our study, most of the patients with infectious granulomatous lesions were in the 3rd and 4th decade (38.5%) followed by 19.2% patients in the 1-20 years age group. Manandhar and Moorthy et al., had similar observations with majority of their cases lying in 20-30 year age group [7,11]. Many researchers have found 4th decade to be commonest age group involved. In the literature, the youngest cases have been found at less than ten years. Six of our paucibacillary cases were in this age group, the youngest being of four years age. The sex distribution pattern of leprosy revealed a male preponderance of 79.42% compared to 20.58 % females (M:F = 3.85). Gautam, Usha Manandhar, and Jaylakshmi also found a male preponderance with 65%, 73% and 75% male patients respectively [3,7,12]. This could be because of more out-patient visits by male patients in hospitals as compared to females due to socio-economic barriers which are rampant in lower and middle strata in all the communities and regions in India. In our study, upper extremity (29% cases) was the most common site involved followed by lower extremity (23% cases) and face (18% cases) while Jha and Karki from Pakistan in their study found neck to be the commonest site [13]. Overall, leprosy showed a good clinico-pathological agreement. Amongst the subtypes, TT and BT showed a clinico-pathological agreement in 72.7% and 56.6% cases respectively. While in BB, BL and LL lesions the clinico-pathological agreement was seen in 33.3%, 30.7% and 35.2% cases respectively [Table/Fig-13]. Chauhari, Nadkarni, Kalla, Jerath VP, Kar PK, and Mathur et al., found a good clinico-pathological correlation (in more than 75% cases) in TT and LL subtypes [9,14–18] while Moorthy et al., and Sharma et al., found maximum clinico-pathological correlation at lepromatous pole only (80% and 75.8% cases respectively) [11,19] [Table/Fig-14]. The polar forms of leprosy show a characteristic distribution and morphology of the lesions with nerve involvement, however differentiating TT from BT and BL from LL is difficult clinically because of considerable overlapping features with only minor clinical differences. The characteristic histopathology of polar forms with ease at demonstrating the lepra bacilli at lepromatous pole allows a better clinico-histological correlation. The disparity in borderline forms is observed because of immunological instability and conversion of one form to another. Thus it depends on time of taking the biopsy and the lesion biopsied.
Clinico-pathological correlation of leprosy cases in present study.
| HISTOPATHOLOGICAL DIAGNOSIS |
|---|
| Sr. No. | Clinical diagnosis | TT | BT | BB | BL | LL | HH | TYPE I | ENL | LV | GA | Dermatitis | No. of Cases |
|---|
| 1 | TT | 8 (72.73%) | 3 (27.27%) | - | - | - | - | - | - | - | - | - | 11 |
| 2 | BT | 21 (27.6%) | 43 (56.6%) | 1 (1.32%) | 5 (6.58%) | - | - | 2 (2.6%) | 1 (1.32%) | 1 (1.3%) | - | 2 (2.63%) | 76 |
| 3 | BB | - | 1 (33.33%) | 1 (33.3%) | 1 (33.33%) | - | - | - | - | | - | - | 3 |
| 4 | BL | 3 (23.08%) | 2 (15.38%) | 1 (7.69%) | 4 (30.77%) | 3c(23.0%) | - | - | - | - | - | - | 13 |
| 5 | LL | 1 (5.88%) | 1 (5.88%) | 4 (23.53%) | 4 (23.53%) | 6 (35.2%) | 1 (5.9%) | - | - | - | - | - | 17 |
| 6 | HH | - | - | - | - | 2 (50%) | 1 (25%) | - | 1 (25%) | - | - | - | 4 |
| 7 | Hansen | 3 (42.86%) | 2 (28.57%) | - | 1(14.29%) | - | - | - | - | - | 1 (14.3%) | - | 7 |
| 8 | Type 1 | 6 (15%) | 7 (17.50%) | - | 5 (12.50%) | 2 (5.00%) | - | 18 (45%) | - | - | 1 (2.5%) | - | 40 |
| 9 | ENL,LL | - | - | - | 2 (8.33%) | 4 (16.7%) | - | 1 (4.2%) | 17 (70.8%) | - | - | - | 24 |
| 10 | IL | 1 (50%) | 1 (50%) | - | - | - | - | - | - | - | - | - | 2 |
| 11 | Relapse | 2 (20%) | 2 (20%) | - | 2 (20%) | 3 (30%) | - | - | 1 (10%) | - | - | - | 10 |
| Total | 45 (21.7%) | 62 (29.9%) | 7 (3.38%) | 24 (11.6%) | 20 (9.7%) | 2 (0.96%) | 21 (10%) | 20 (9.7%) | 1 (0.5%) | 2 (0.9%) | 2 (0.97%) | 207 |
Comparison of clinico-pathological correlation with different studies.
| Author name | Year of study | TT | BT | BB | BL | LL |
|---|
| Nadkarni [14] | 1999 | 88% | 80.70% | 69.90% | 81.30% | 95.30% |
| Kalla et al., [15] | 2000 | 75.60% | 44.20% | 37% | 43.70% | 76.70% |
| Moorthy et al., [11] | 2001 | 46.15% | 66.54% | 50% | 70% | 80% |
| Sharma et al., [19] | 2008 | 47.37% | 53.01% | 37.35% | 58.82% | 75.86% |
| Gautam k et al., [3] | 2008 | 33% | 72.20% | 100% | | 50% |
| Mathur MC et al., [18] | 2009 | 73.2% | 89.74% | 64.7% | 72.4% | 95.2% |
| Manandhar [7] | 2010 | 24% | 63.15% | 0% | 57.14% | 57.14% |
| Current study | 2011 | 72.73% | 56.60% | 33.30% | 30.77% | 35.20% |
Out of 26 histopathologically proven cases of cutaneous TB, lupus vulgaris (53.85%), scrofuloderma (15.38%), TBVC and papulonecrotic tuberculid (11.54% each) were the commonest. Similary Amanjit Bal, found lupus vulagris and scrofuloderma as most common skin TB while T Kaur et al., Zafar et al., and Dwari et al., found TBVC to be the second most common diagnosis after lupus vulgaris [1,4–6,20]. The presentation of skin TB depends on the organism which is mainly M. tuberculosis, the route of infection (endogenous or exogenous), the immune status and previous sensitization with tuberculosis. Thus skin can react in many different ways to a single disease agent giving rise to such different presentations [1].
Most commonly affected was lower extremity. Lupus vuagaris and TBVC were more common on lower limbs. Scrofuloderma affected neck, axilla and lower back. Lupus vulgaris results from haematogenous, lymphatic or contiguous spread while scrofuloderma results from contiguous involvement of skin overlying tuberculosis in deeper structures, for example lymph node, bone or joint that’s why it was common in neck, axilla and back. TBVC is inoculation tuberculosis in an immunocompetent host so more chances of affecting lower limbs.
The most commonly affected age group in our study was 21-30 years with 35.7% cases of lupus vulgaris, 50% cases of scrofuloderma and 66% cases of TBVC seen in this age group. In our study, lupus vulgaris was found equally in both sexes (seven cases each) where as Scrofuloderma, TBVC and papulonecrotictuberculid showed male preponderance. In their study Zafar et al., found more females being affected by all forms of tuberculosis [5,6]. The most commonly affected age group in our study was 16-30 years which is in concordance with the study by Kaur T et al., and Dwari et al., [4,20]. Maximum clinico-pathological correlation was found in cases of scrofuloderma (100%) followed by lupus vulgaris (58.83%) and TBVC (50%) [Table/Fig-14].
Multidrug resistant TB strains and AIDS epidemic have lead to a world-wide increase in tuberculosis more so in poverty-struck Indian and African subcontinent due to poor nutrition, non-availability of diagnostic aids and treatment, overcrowding and ignorance about the disease. In spite of the BCG vaccinations tuberculosis takes a heavy toll on poor socioeconomic strata specially children and young adults. It is alarming to all officials dealing with control and management of tuberculosis.
Increase incidence of IGDS specially leprosy and tuberculosis at our center could be because of ease of access to patients leading to more attendance at the out-patient department and government funded control programs leading to free facility of skin biopsies. Ours being a state government supported tertiary care referral center catering to poor and middle class social strata of Mumbai with a population of 150 million with many immigrants from Bihar, Uttar Pradesh, and other states of India leading to massive overcrowding and poor hygienic conditions.
Thus increased awareness among the people with efforts from central government, state government, institutions, social workers working for the poor socioeconomic strata, treating dermatologists, leprologists, DOTS center health care workers, increased diagnostic acumen among pathologists with good facilities at pathology and microbiology laboratories like PCR, fast microbiological cultures are few of the steps needed to help uproot the infectious lesions including IGDS.
Conclusion
Increased incidence of IGDS specially leprosy and skin TB needs an all round approach with a good health care team, epidemiologists, control program execution and better access of health services to curb the increased burden of these crippling diseases. Communication between clinicians and pathologists with strict adherence to clinical criteria while choosing the proper site of biopsy, age of lesion, nature and depth of biopsy and knowledge of treatment and immunological status with coexistence of other co-morbidities like HIV and AIDS is a must. Similarly, adequate and good pathological sections, special stains, diagnostic acumen, awareness of differential diagnosis and histopathological features of various type of lesions with a good correlation with the clinical findings to arrive at an accurate diagnosis will help the dermatologists and leprologists to provide adequate treatment to the ailing patients.
[1]. Bal A, Mohan H, Dhami GP, Infectious granulomatous dermatitis: a clinicopathological studyIndian J Dermatol 2006 51(3):217-20. [Google Scholar]
[2]. Dhar S, Histopathological features of granulomatous skin diseases: An Analysis of 22 skin biopsiesIndian Journal Dermatol 2002 47(2):88-90. [Google Scholar]
[3]. Gautam K, Pai RR, Bhat S, Granulomatous lesions of the skinJournal of Pathology of Nepal 2011 1:81-86. [Google Scholar]
[4]. Kaur T, Thakur A, Pandey K, Malhotra SK, Puri KPS, Cutaneous TB profile in North West Punjab, India: a retrospective data analysisOur Dermatol Online 2013 4(4):458-61. [Google Scholar]
[5]. Zafar MN, Memon MA, Agha MA, Agha SA, Hashim Y, Muhammad TM, Pattern of cutaneous tuberculosis as identified by morphological study of skin lesions at Jinnah postgraduate medical center, KarachiGomal Journal of Medical Sciences 2010 8(1):44-49. [Google Scholar]
[6]. Zafar MN, Sadiq S, Memon MA, Morphological study of different granulomatous lesions of the skinJournal of Pakistan Association of Dermatologists 2008 18:21-28. [Google Scholar]
[7]. Manandhar U, Adhikari RC, Sayami G, Clinico-histopathological correlation of skin biopsies in leprosyJournal of Pathology of Nepal 2013 3:452-58. [Google Scholar]
[8]. Pandya AN, Tailor HJ, Clinicohistopathological correlation of leprosyIndian J Dermatol Venereol Leprol 2008 74:174-76. [Google Scholar]
[9]. Chauhari B, Mehta RP, Clinico-Histopathological Correlation in LeprosyInternational Journal of Scientific Research 2012 1(5):104-05. [Google Scholar]
[10]. Jindal N, Shankar V, Tegta GR, Gupta M, Verma GK, Clinicoepidemilogical trends of leprosy in Himachal Pradesh: a five year studyIndian J Lepr 2009 81:175-79. [Google Scholar]
[11]. Moorthy BN, Kumar P, Chatura KR, Chandrasekhar HR, Basavaraja PK, Histopathological correlation of skin biopsies in leprosyInd J DermatolVenereol Leprol 2001 67:299-301. [Google Scholar]
[12]. Jayalakshmi Histopathology of skin lesions in leprosyMalaysian J Pathol 1980 3:39-45. [Google Scholar]
[13]. Jha R, Karki S, Limitations of Clinico-histopathological Correlation of Skin Biopsies in LeprosyJ Nepal Health Res Counc 2010 8(16):40-43. [Google Scholar]
[14]. Nadkarni NS, Rege VL, Significance of histopathological classification in leprosyInd J Lepr 1999 71:325-32. [Google Scholar]
[15]. Kalla G, Salodkar A, Kachhawa D, Clinical and histopathological correlation in leprosyInt J Lepr 2000 68:184-85. [Google Scholar]
[16]. Jerath VP, Desai SR, Diversities in clinical and histopathological classification of leprosyLeprosy in India 1982 54:130-34. [Google Scholar]
[17]. Kar PK, Arora PN, Clinicopathological study of macular lesions in leprosyIndian J Lepr 1994 66:435-41. [Google Scholar]
[18]. Mathur MC, Ghimire RBK, Shrestha P, Kedia SK, Clinicohistopathological correlation in leprosyKathmandu Univ Med J 2011 36(4):248-51. [Google Scholar]
[19]. Sharma A, Sharma RK, Goswsami KC, Bardwaj S, Clinico-histopathological correlation in leprosyJK Science 2008 10:120-23. [Google Scholar]
[20]. Dwari BC, Ghosh A, Paudel R, Kishore P, A clinicoepidemiological study of 50 cases of cutaneous tuberculosis in a tertiary care teaching hospital in Pokhara, NepalIndian J Dermatol 2010 55(3):233-37. [Google Scholar]