Comparative Efficacy of Intrathecal Bupivacaine Alone and Combination of Bupivacaine with Clonidine in Spinal Anaesthesia
Ajay Wahi1, Amanjot K. Singh2, Kartik Syal3, Ajay Sood4, Jyoti Pathania5
1 Assistant Professor, Department of Neuroanaesthesia, MMIMSR, Mullana, Ambala, Haryana, India.
2 Assistant Professor, Department of Community Medicine, MMIMSR, Mullana, Ambala, Haryana, India.
3 Assistant Professor, Department of Anaesthesia, IGMC, Shimla, Himachal Pradesh, India.
4 Professor, Department of Anaesthesia, IGMC, Shimla, Himachal Pradesh, India.
5 Professor, Department of Anaesthesia, IGMC, Shimla, Himachal Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ajay Wahi, A-10, A Block, MMU Campus, Mullana, Ambala, Haryana-133207, India.
E-mail: ajaywahi81@yahoo.com
Introduction
Clonidine is an α2 agonist agent that has been used as an adjuvant to local anaesthetics in regional anaesthesia.
Aim
This study compared two combinations of bupivacaine and clonidine with bupivacaine alone for surgeries below the level of umbilicus in spinal anaesthesia.
Materials and Methods
We conducted a randomized double blind study on 90 patients of ASA I and ASA II aged 20-60 years, 30 in each group, undergoing surgery below the level of umbilicus in spinal anaesthesia. For intrathecal block, Group 1 received bupivacaine hydrochloride 12.5mg (2.5ml) in 8% dextrose (0.5% sensorcaine heavy) + 1ml (150μg) of preservative free clonidine. Group 2 received bupivacaine hydrochloride 12.5mg (2.5ml) in dextrose (0.5% sensorcaine heavy) + 0.5ml (75μg) of preservative free clonidine + 0.5ml of normal saline to make the volumes of all the groups same. Group 3 received bupivacaine hydrochloride 2.5ml in 8% dextrose (0.5% sensorcaine heavy) + 1ml of normal saline to make the volumes of all the groups same. Heart rate, NIBP, oxygen saturation and respiratory rate were monitored. The onset and duration of sensory block, the highest dermatomal level of sensory block, motor block, time to complete motor block recovery and duration of spinal anaesthesia were recorded.
Statistical Analysis
The data of the study was recorded in the record chart and results were evaluated using statistical tests (ANOVA test, post-hoc turkey hsd test, paired t-test and chi-square test).
Results
Demographic data, the incidence and duration of bradycardia were comparable amongst the groups. The duration of sensory and motor block were greatest in group 1, followed by group 2 and group 3 (p <0.01). Decrease in the systolic blood pressure of group 2 and group 3 was noted as compared to group 1. No significant sedation or respiratory depression was observed in any group.
Conclusion
Addition of clonidine to bupivacaine intrathecally is although a reliable method to prolong spinal anaesthesia but close monitoring for hypotension is desirable.
Adjuvant, α2 agonist, ASA 1&2, Bromage scale, Lower abdominal surgery, Subarachnoid block
Introduction
Spinal anaesthesia is being practiced safely and effectively since many years. Like most regional anaesthesia techniques it is easily administered and gives excellent surgical conditions [1,2]. But spinal anaesthesia using local anaesthetics has a limited duration of action that may result in conversion to general anaesthesia for completion of surgery. Similarly, combined spinal epidural anesthesia has also been tried but it is technically more challenging [3], may not provide good surgical conditions and has its own side effects. Therefore, continuous research is going on to improve upon this most used regional anaesthetic technique. Tamsen and Gordh proved safety of parenteral preparation of clonidine for intrathecal use in humans [4]. Although, even large doses of clonidine intrathecally were inadequate to produce surgical anaesthesia [5], it repeatedly prolonged sensory and motor blockade when used as an adjuvant to local anaesthetics. Various studies used clonidine as adjuvant in different doses and with different local anaesthetics, but they have yielded conflicting results [5–7].
Aim
In this comparative study, the effects of bupivacaine alone and bupivacaine in combination with two different doses of clonidine were evaluated in patients undergoing surgery below the level of umbilicus in spinal anaesthesia.
Materials and Methods
After the approval by the ethical committee of Indira Gandhi Medical College, Shimla, we conducted a randomized double blind study on 90 patients of either sex belonging to ASA physical status I and II, scheduled for surgery below the level of umbilicus in spinal anaesthesia over a period of six months. The patients with bradyarrythmia, atrioventricular heart block, having epilepsy or depressive neurological disorder and other relative or absolute contraindications to spinal anaesthesia were excluded from the study. All the patients were informed of the procedure to be carried out on them in the operative and the recovery room and written consent was obtained. The patients were allotted to either of the 3 study groups of 30 patients each as per random number list. To achieve a double blind study, the trial drug was prepared, coded and administered by an anesthetist not otherwise participating in the study. Both, the patient as well the investigator observing the results, were blind to the test drug by giving serial numbers to the patients which were decoded at the end of the study. All observations were made by the same observer to eliminate subjective error.
All routine investigations were done prior to the surgery. All the patients were kept nil per oral for at least six hours prior to the surgery.
anaesthesia
Premedication in the form of tablet alprazolam 0.25mg was given on the night before the operation. After shifting the patient to operation theatre, baseline parameters like ECG, HR, NIBP, RR, peripheral oxygen saturation (spO2) were recorded by the candidate who was blinded to the adjuvant drug used. In the operation theatre intravenous line was secured in all patients and 5ml/kg of isotonic normal saline was given before giving subarachnoid block. Monitoring of the heart rate, ECG, NIBP and oxyhaemoglobin saturation was established. Under all aseptic conditions subarachnoid puncture was performed using midline lumbar approach, with patient in sitting position using 26 gauge Quincke needle in L3-L4 interspace and after confirmation of the free flow of CSF, drugs were given according to the three groups in a double blind way.
The various treatment groups were as under:
Group 1 - 2.5ml of 0.5% heavy bupivaicaine + 1ml (150μg) of clonidine.
Group 2 – 2.5ml of 0.5% heavy bupivacaine + 0.5ml of normal saline + 0.5ml (75μg) of clonidine.
Group 3 – 2.5ml of 0.5% heavy bupivacaine + 1ml of normal saline.
The patients were then turned supine with no head tilt. Baseline values for monitored variables were taken. Heart rate, NIBP, oxyhaemoglobin saturation was monitored every minute for the first 10 minutes, every 5 minutes for the next 20 minutes and then at an interval of 15 minutes for one hour after subarachnoid block. Respiratory rate, level of sedation (Ramsay’s scoring), grade of motor block (Bromage scale) and level of sensory block (pin prick test) were monitored every 1 hour after injecting the drug until end of the study. Level of sensory block was seen by pin prick method after 7 minutes of injecting the drug. Time taken for the onset of action was noted from the time of injecting the drug into subarachnoid space to complete analgesia at the level of lower border of umbilicus. Duration of sensory block was calculated as the time taken for regression to two lower levels compared to that at the onset, which was taken as end point of the study. Duration of motor block was calculated as the time from the onset of action to no block on Bromage scale. A fall of more than 30% in systolic arterial pressure of baseline value or a reading of less than 90 mm Hg was treated with an intravenous bolus of 6 mg of mephenteramine. Any episode of bradycardia (heart rate < 50/min) was treated with 0.6 mg of i.v. atropine.
Statistical Analysis
The data of the study was recorded in the record chart and results were evaluated using statistical tests (ANOVA test, post-hoc turkey hsd test, paired t-test and chi-square test).
Results
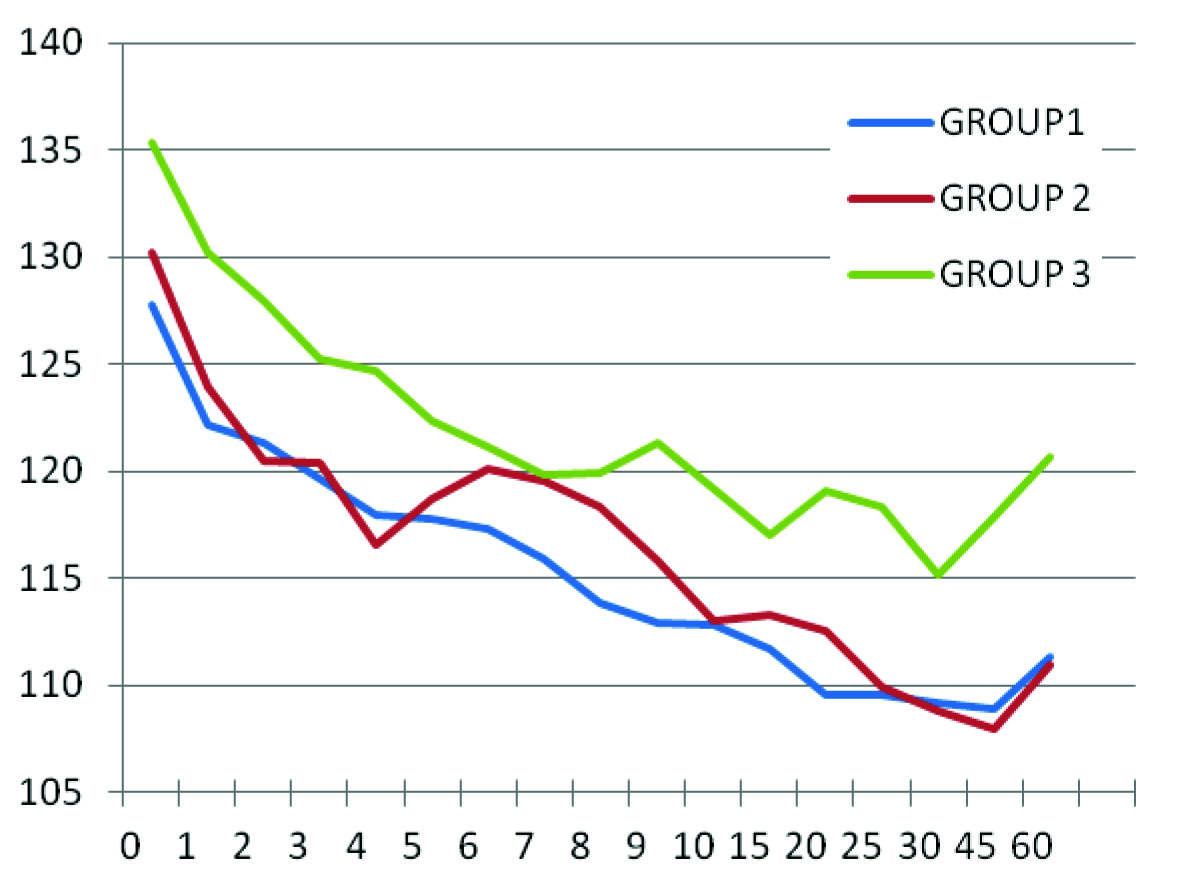
The age, sex and weight were comparable in all the three groups as shown in [Table/Fig-1]. The difference between baseline parameters was also insignificant. The time taken for the onset of action and the level of sensory block achieved was comparable in all groups. Although, the heart rate decreased significantly in group 2 and group 3 as compared to their baseline values, but it remained comparable when group 2 was compared to group 3 as shown in [Table/Fig-2]. Incidence of bradycardia was statistically insignificant. The systolic blood pressure showed significant decrease in all the groups as compared to the baseline value. The intergroup comparison showed significant decrease in the systolic blood pressure of group 2 and group 3 from 25th minute to 60th minute as compared to group 1 as shown in [Table/Fig-3]. There was no significant difference noted in between group 2 and group 3. However, the incidence of hypotension requiring intervention was significantly more in group 2 as compared to group 1 and group 3. Other monitored parameters were comparable in all the groups throughout the study. No other untoward side effects were noticed in this study. The duration of sensory and motor block of the three groups are shown in [Table/Fig-4].
Demographic Characteristics.
| Characteristics | Mean ±S.D. |
|---|
| Group1 | Group2 | Group3 |
|---|
| Age(years) | 34.87±9.7 | 35.53±11.26 | 32.2±9.83 |
| Weight(kg) | 55.7±6.26 | 56.2±5.52 | 54.83±6.52 |
| Sex | Males 16Females 14 | Males 18Females 12 | Males 16Females 14 |
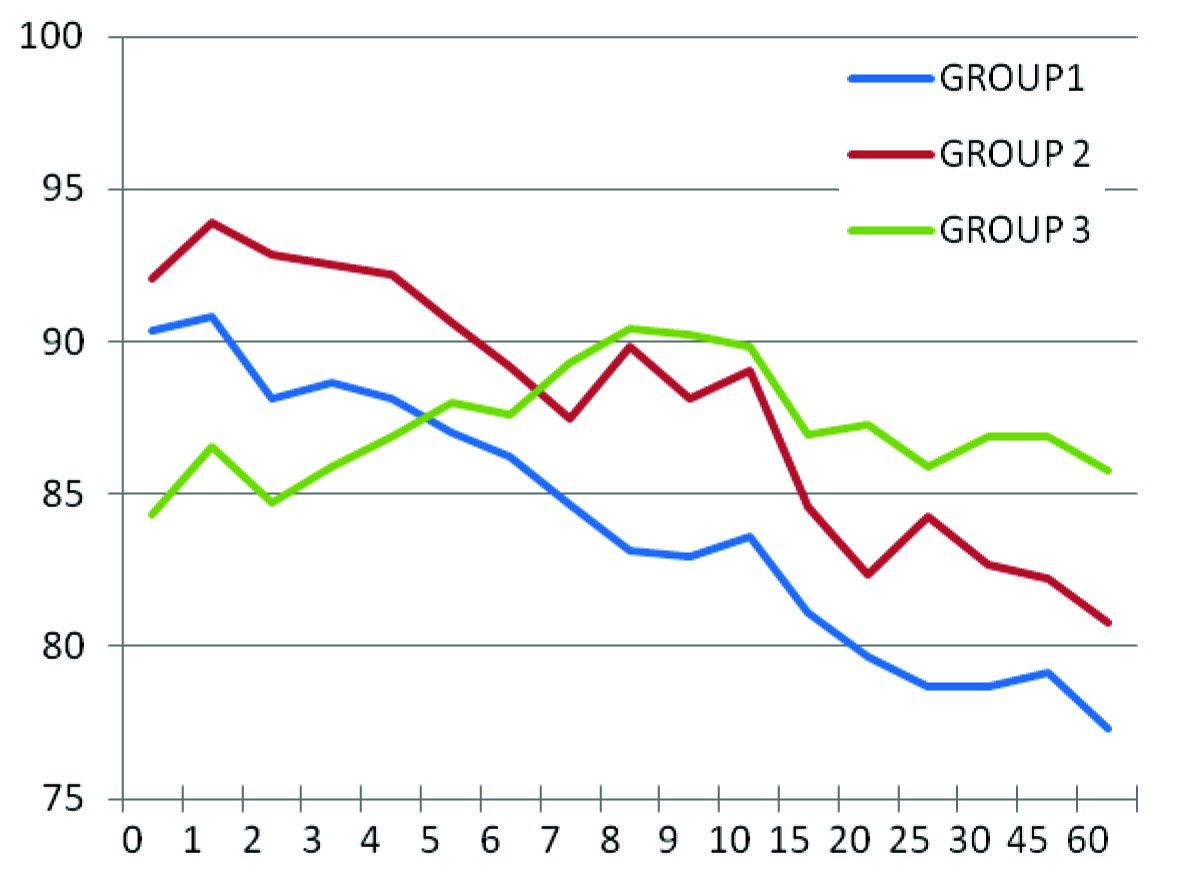
Comparison of Mean Heart Rates in Groups 1, 2 & 3.
Comparison of Systolic Blood Pressure in Groups 1, 2 & 3.
Characteristics of Sensory and Motor Block.
| Group 1 | Group 2 | Group 3 |
|---|
| Onset of action | 4.67±1.84 | 4.27±1.48 | 5.03±1.27 |
| Duration of sensory block (min) | 125±25.01* | 228±39.86* | 320±39.65* |
| Duration of motor block (min) | 112.50±25.15* | 198±50.2* | 232±34.28* |
*p<0.01
Discussion
These results showed that the duration of sensory as well as motor block increased significantly in a dose dependent manner in groups in which clonidine was administered than in the control group. These findings support the observation made by Niemi et al., and Strebel et al., [7,8]. However, Benhamou and co-workers did not find prolongation of anaesthesia in their study [9], which in all probability must be due to the use of repeated boluses of intravenous fentanyl and epidural lignocaine whenever intraoperative analgesia was insufficient. This intervention may have lead to delay in regression of sensory blockade even in control group. Also, all the patients in the study were undergoing elective caesarean section that required consistently higher level of sensory blockade. Haemodynamically, heart rate was comparable in all the groups but as compared to its respective baseline value in group 3 and group 2, the heart rate decreased significantly from 7th and 15th minute, respectively. This is explained by the fact that following intrathecal injection, clonidine is rapidly redistributed into plasma with a t1/2 alpha of 7.3 minutes with peak arterial levels at 10 minutes and venous level within 30-45 minutes [10]. Clonidine after systemic absorption decreases heart rate by enhancing baseline vagal activity and by a direct action on the heart. Incidence of bradycardia was 2, 1 and 0 in group 1, group 2 and group 3, respectively. These findings coincide with that of De Negri et al., and BS Sethi et al., [11,12]. However, Niemi et al., reported a significant reduction in the heart rate [7]. That may be due to the greater dose of hyperbaric bupivacaine (15 mg) as well as clonidione (3 μg/kg). Systolic blood pressure in clonidine groups exhibited a decrease as compared to the control group. Similar trend was observed by Niemi et al., [7]. But the incidence of hypotension was more in groups 3 and 2 than group 1. Exact explanation could not be found but JC Eisenach reported increased haemodynamic stability with use of intermediate to large doses of clonidine in sheep that resulted in activation of both α1 and α2 receptors [13]. A detailed dose response study in humans may indicate a favourable intermediate dose. Haemodynamic derangement occurs in clonidine groups after 20 minutes as compared to control group and stabilizes near 60 minutes. NIBP monitoring for some more duration may clear the exact pattern of recovery to baseline values. Other side effects such as sedation and dry mouth were not found in this study as they were found in the study by Niemi et al., and BS Sethi et al., [7,12]. The former used diazepam per os to their patients on the morning of surgery and the latter used double the maximum dose of clonidine used in this study.
Conclusion
This study concludes that intrathecal clonidine as an adjuvant to bupivacaine is an effective and convenient method to prolong spinal anaesthesia in a dose dependent manner for prolonged surgeries below the level of umbilicus with a limitation of close monitoring of NIBP and need of further studies with different doses to find the ideal dose of clonidine.
*p<0.01
[1]. McConachie I, Anaesthesia for the high risk patient 2002 CambridgeCambridge University Press:70-4. [Google Scholar]
[2]. Larson MD, History of Anaesthetic Practice. In: Miller RDMiller’s Anaesthesia 2005 Sixth EditionPhiladelphiaElsevier:25-26. [Google Scholar]
[3]. Kestin L, A statistical approach to measure the competence of anaesthetic trainees at practical proceduresBr J Anaesthesia 1995 75:805-09. [Google Scholar]
[4]. Tamsen A, Gordh T, Clonidine is not neurotoxicLancet 1984 ii:876 [Google Scholar]
[5]. Filos KS, Goudas LC, Patroni O, Polyzou V, Haemodynamic and analgesic profile after intrathecal clonidine in humans: a dose response studyAnesthesiology 1994 81:591-601. [Google Scholar]
[6]. Dobrydjnov I, Axelsson K, Samarutel J, Holmstrom B, Postoperative pain relief following intrathecal bupivacaine combined with intrathecal or oral clonidineActa Anaesthesiol Scand 2002 46:806-14. [Google Scholar]
[7]. Niemi L, Effects of intrathecal clonidine on duration of bupivacaine spinal anaesthesia, haemodynamics and postoperative analgesia in patients undergoing knee arthroscopyActa Anaesthesiol Scand 1994 38:724-28. [Google Scholar]
[8]. Strebel S, Gurzeler JA, Schneider MC, Aechbach A, Kindler CH, Small dose intrathecal clonidine and isobaric bupivacaine for orthopedic surgery: a dose - response studyAnesth Analg 2004 99(4):1231-38. [Google Scholar]
[9]. Benhamou D, Thorin D, Brichant JF, Dailland P, Milon D, Shneider M, Intrathecal clonidine and fentanyl with hyperbaric bupivacaine improves analgesia during cesarean sectionAnesth Analg 1998 87:609-13. [Google Scholar]
[10]. Eisenach JC, De Kock M, Klimscha W, Alpha2-adrenergic agonists for Regional Anaesthesia: A Clinical Review of Clonidine (1984-1995)Anaesthesiology 1996 85:655-74. [Google Scholar]
[11]. De Negri P, Borelli F, Salvatore R, Visconti C, De Vivo P, Mastronardi P, Spinal anesthesia with clonidine and bupivacaine in young humans: interactions and effects on the cardiovascular systemMinerva Anestesiol 1997 63(4):119-25. [Google Scholar]
[12]. Sethi BS, Samuel M, Sreevastava D, Efficacy of analgesic effects of low dose intrathecal clonidine as adjuvant to bupivacaineIndian Journal of Anaesthesia 2007 51(5):415-19. [Google Scholar]
[13]. Eisenach JC, Tong C, Site of haemodynamic effects of intrathecal α2-adrenergic agonistsAnesthesiology 1991 74:766-71. [Google Scholar]