The present study was designed to see whether such variations in the proportion of ER and Ki-67 expression can help distinguish three broad categories of breast lesions namely benign breast disease, proliferative breast disease and malignant breast disease.
Materials and Methods
This is a prospective study to evaluate ER and Ki-67 expression in breast lesions. The study included 52 cases which were clinically and cytologically diagnosed as either benign or malignant, and, underwent surgery and further histopathological evaluation. Among these cases which were initially diagnosed by Haematoxylin and Eosin (H&E) stained tissue sections, 15 cases from each of the benign, proliferative and malignant breast disease category were taken and were studied by using immunohistochemistry using monoclonal antibody to Estrogen Receptor (ER) and proliferation associated antigen (Ki-67) on two separate sections for each individual case. Other uncommon subtypes of breast cancer like lobular carcinoma and medullary carcinoma were excluded from the study.
The percentage of ER positive and Ki-67 positive cells was calculated on the respective sections and expressed as a proportion. A minimum of 200 cells per sections were counted for both ER positivity and Ki 67 positivity and expressed as percentage cells. The cells showing distinctive brown staining of the nuclei were counted as positive [Table/Fig-1,2]. The ER+/Ki-67+proportion was then calculated for each case using the following formula:
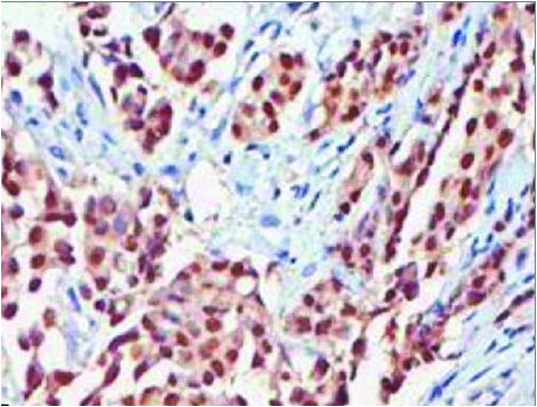
Microphotograph of invasive ductal carcinoma showing positive immunostaining for estrogen receptor (ER); 40X.
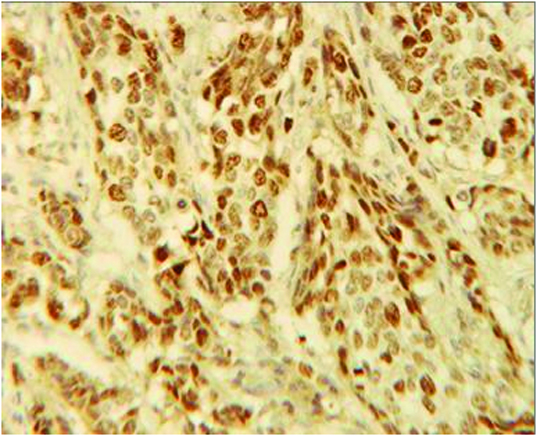
Microphotograph of invasive ductal carcinoma showing positive immunostaining for Ki 67 (ER); 40X.

The clinical and diagnostic significance of variations in the ER+/Ki-67+ proportion between the three categories of disease mentioned above was analyzed by applying appropriate statistical methods. Comparison was carried out by using student’s t-test with p-value < 0.05 as significant. Pearson’s coefficient of correlation was used to correlate ER and Ki-67 positivity within each category.
Results
Patient characteristics are mentioned in [Table/Fig-3].
| Benign Breast Disease | Proliferative Breast Disease | Malignant Breast Disease |
|---|
| Age (in Years) |
| Mean | 30.8 | 42.4 | 48.67 |
| Range | 16 – 58 | 25 – 50 | 30 – 67 |
| Laterality |
| Right | 8 (53.33%) | 9 (60.0%) | 8 (53.33%) |
| Left | 7 (46.67%) | 6 (40.0%) | 7 (46.67%) |
| ER + (%) |
| Mean ± SD | 15.83 ± 7.94 | 20.23 ± 8.87 | 21.3 ± 8.25 |
| Range | 5 – 39 | 10 – 37 | 7 – 36 |
| Ki 67 + (%) |
| Mean ± SD | 20.02 ± 4.38 | 22.70 ± 7.70 | 16.83 ± 8.59 |
| Range | 13.8 – 30 | 12 – 34.5 | 4 – 34.5 |
| ER +/ Ki 67 + |
| Mean ± SD | 0.81 ± 0.43 | 0.87 ± 0.13 | 1.42 ± 0.58 |
| Range | 0.26 – 2.17 | 0.76 – 1.09 | 0.88 – 2.79 |
Benign breast disease (Category 1)
A total of 15 cases with histopathological diagnosis of fibroadenoma, fibroadenosis, fibrocystic disease and benign breast aspirate were studied and ER+/Ki-67+ proportion was calculated for each case. This was done to generate a data for control and comparison with proliferative and malignant breast disease.
The percentage of ER+ cells ranged from 5% to 39% with a mean of 15.83 and a standard deviation of 7.94. The percentage of Ki-67+ cells ranged from 13.8% to 30% with a mean of 20.02 and a standard deviation of 4.38. The ER+/Ki-67+ proportions ranged from 0.26 to 2.17 with a mean of 0.81 and a standard deviation of 0.43.
Proliferative breast disease (Category 2)
A total of 15 cases with a histopathological diagnosis of proliferative breast disease, proliferative breast disease with marked atypia, ductal hyperplasia and epithelial atypia were studied and ER+/Ki-67+ proportions was calculated for each case.
The percentage of ER+ cells ranged from 10-37% with a mean of 20.23% and a standard deviation of 8.87. The percentage of Ki-67+ cells ranged from 12-34.5% with a mean of 22.70% and a standard deviation of 7.70. The ER+/Ki-67+ proportions ranged from 0.76-1.09 with a mean of 0.87 and a standard deviation of 0.13.
Malignant breast disease (Category 3)
A total of 15 cases with a histopathological diagnosis of infiltrating ductal carcinoma were studied and ER+/Ki-67+ proportion was calculated for each case.
The percentage of ER+ cells ranged from 7-36% with a mean of 21.3% and a standard deviation of 8.25. The percentage of Ki-67+ cells ranged from 4-34.5% with a mean of 16.83 and a standard deviation of 8.59. The proportion of ER+/Ki-67+ cells ranged from 0.88-2.79 with a mean of 1.42 and a standard deviation of 0.58.
Comparison between groups
Student t-test was applied to the mean proportions of the three categories and the results were tabulated and shown in [Table/Fig-4]. A statistically significant difference in ER+/Ki-67+ proportions was observed between proliferative breast disease category and malignant breast disease category and also between benign breast disease category and malignant breast disease category (p<0.05). However, no significant difference was observed in benign breast disease category and proliferative breast disease category (p> 0.05).
Comparison between categories (Student t-test).
| Categories | t-value | p-value |
|---|
| 1-2 | -0.52 | >0.05 |
| 1-3 | -3.26 | <0.05 |
| 2-3 | -3.56 | <0.05 |
Correlation between ER+ and Ki-67+
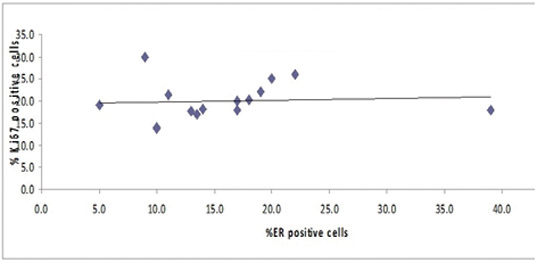
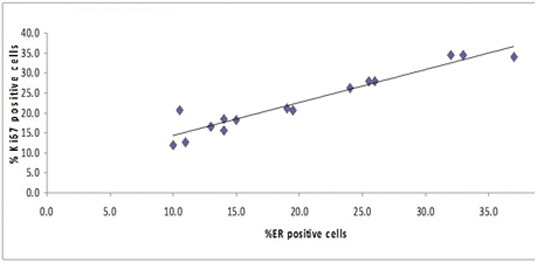
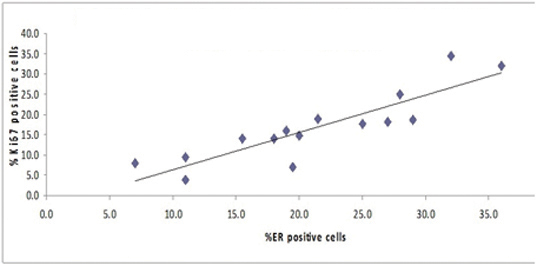
ER+ and Ki-67+ positivities within each category were correlated using pearson’s coefficient of correlation and the results were tabulated in [Table/Fig-5]. A significant correlation was observed in proliferative breast disease and malignant breast disease categories. However, no significant correlation was observed in benign breast disease category [Table/Fig-6,7 and 8].
Correlation between ER+ and Ki-67+.
| Categories | r-value | p-value |
|---|
| Benign breast disease | 0.0797 | >0.1 |
| Proliferative breast disease | 0.9587 | <0.001 |
| Malignant breast disease | 0.8804 | <0.001 |
Relationship between % ER +ve cells and % Ki-67+ve cells (Benign Breast Disease).
Relationship between % ER +ve cells and % Ki 67 +ve cells (Proliferative Breast Disease).
Relationship between % ER +ve cells and % Ki 67 +ve cells (Malignant Breast Disease).
Discussion
Breast cancer is one of the most common malignancies in women all over the world [1]. The natural history of breast cancer is characterized by long duration and marked heterogeneity [2]. The exact aetiology and events of tumourigenesis that trigger the development of breast cancer de novo as well as from the ‘precursor’ lesions are yet to be elucidated. However, the study by Dupont and Page has shown a moderate increase in the risk of subsequent cancer for patients with benign breast disease and proliferative breast disease with and without atypia [4]. Carter et al., in a prospective study of approximately 17,000 patients with benign breast disease among participants in the breast cancer detection demonstration project (BCDDP), found a 3 fold increase in the risk of cancer subsequent to atypical hyperplasia [5].
With the advent of new ancillary techniques like immunochemistry, DNA flow-cytometry, image cytometry etc., much advancement were offered in the past to elucidate the pathogenesis of breast cancer. Other prognostic markers which have gained popularity include estrogen receptor status and proliferation associated cell markers like Ki-67 [3]. Various studies on the independent clinical validity of different parameters have shown contradictory results, and no single parameters has consistently predicted prognosis. However, a combination of parameters have shown better results. Hemachandran et al., found a significant correlation between cytological and histological grading when cytomorphology is combined with image morphometry [6].
Proliferation index rates and ER status are thus, two of the important prognostic factors which can predict overall and disease free survival in breast cancer [7,8]. So, knowledge of ER status, Ki-67 score and their proportion (ER+/Ki-67+) would be helpful to a certain extent in the treatment and management of breast cancer and proliferative lesions.
The study by Shoker et al., has shown Ki-67 positive cells were also present within all benign lesions studied, ranging from 1.5% of cells in sclerosing adenosis to 4.4% in fibroadenomas [7]. Lesser variability in the expression of Ki-67 and ER within similar lesions is observed. The study by Clarke et al., also showed a significant co-expression of estrogen receptor expression and cell proliferation antigen in invasive carcinomas [8].
These studies were done on histological sections and have shown a loss of the inverse relationship between ER+ and Ki-67+ expression, meaning thereby that the cells express both ER and Ki-67 on them. In other words, these studies suggest that with a rising grade in the lesion the ER+ cells gain greater autonomy from regulatory factors and actively proliferate, in contrast to benign or low grade lesions.
Our present study was performed on three categories of breast neoplastic disease i.e. benign breast disease, proliferative breast disease and malignant breast disease. It involved counting of ER+ and Ki-67 cells in each case and calculation of the proportion of these two. The ER+/Ki-67+ proportion was compared between the three categories. The ER+ and Ki-67+ percentages were also calculated for cases within each category to see if there was correlation between the two markers. The actual percentage of ER positivity in the cases included in our study, as also in the studies by Shoker et al., and Clarke et al., was not an important consideration since the ER+/Ki-67+ proportion was the main concern. This is based on the suggestion of a close association between ER positivity and proliferative capacity in the studies by Shoker et al., and Clarke et al., [7,8].
We found, on applying the student t-test, a significant increase in the ER+/Ki-67+ proportion when compared between the benign breast disease category and the malignant breast disease category, and between the proliferative breast disease category and the malignant breast disease category. No significant increase in the ER+Ki-67+ proportion was noted when compared between the benign breast disease category and the proliferative breast disease category. However, on applying the Pearson’s coefficient of correlation for the individual categories, we found a significant correlation between ER+ and Ki-67+ cells even within the proliferative breast disease category, as also within the malignant breast disease category. No such correlation was noted within the benign breast disease category.
These observations suggest that the inverse relationship between ER+ and Ki-67+ cells which characterizes the normal breast, and which also persists in benign breast disease, is progressively lost in proliferative breast disease and further in breast cancer. It also appears that this change is more pronounced when compared between proliferative breast disease category and breast cancer than between benign and proliferative breast disease category.
The possible reason for a more pronounced change in the ER+/Ki-67+ status between the proliferative and malignant disease categories than between the benign and proliferative disease categories is that, perhaps, these events are slow to start with and take time to establish before progressing to a higher grade lesion. This also brings into contention the matter of ER+ sensor cells and their contiguity with the surrounding cells already alluded to above. It is, thus, conceivable that the ER+ sensor cells modulate the proliferative capacity of the surrounding ER positive and ER negative cells, and that growing autonomy of such cells leads to frank malignancy.
Conclusion
In our study, a rising consistent graph in the Pearson coefficient of correlation test from the proliferative breast disease category to the malignant breast disease category may suggest a continuum of disease between these categories and offers a change in the ER+/Ki-67+ status as a possible mechanism.
The present study is a small one, and also the pronounced variability in ER+/Ki-67+ proportions which we observed in the three categories studied needs further investigation by including a large number of cases. This may help determine whether women whose lesions show the highest ER+/Ki-67+ proportion may help predict prognosis, and may have bearing on the overall and disease-free survival rates. These investigations may also include progesterone and progesterone receptor to see how these modify breast carcinogenesis. Such studies may help us understand breast disease in terms of biochemical steps and ultimately shed light on the whole event of carcinogenesis especially hormonally mediated/modulated carcinogenesis.