Inflammatory Myofibroblastic Tumour of Thyroid with its Prominent Spindle Cell Pattern: A Rare Case Report
S. Marylilly1, T. Subachitra2, V. Ramya3
1 Professor, Department of Pathology, Stanley Medical College, Chennai, Tamil Nadu, India.
2 Assistant Professor, Department of Pathology, Stanley Medical College, Chennai, Tamil Nadu, India.
3 Postgraduate, Department of Pathology, Stanley Medical College, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. T. Subachitra, AN 22, TNHB Quarters, Todd Hunter Nagar, Saidapet, Chennai-600015, Tamil Nadu, India.
E-mail: t.subachitra@gmail.com
Inflammatory myofibroblastic tumour of thyroid is very rare. Only 18 cases reported so far. Here we report a case of Inflammatory myofibroblastic tumour with its prominent spindle cell (fibrohistiocytic) pattern in a 61-year-old male patient. The dominant histological pattern in our case was myofibroblastic in contrast to prominent lymphoplasmocytic pattern in other previously reported cases. The tumour was strongly positive for vimentin, Anaplastic lymphoma kinase and showed focal positivity for Smooth Muscle Actin. The patient was treated with total thyroidectomy and he is comfortable after surgery.
Anaplastic lymphoma kinase, Immunohistochemistry, Lymphoplasmocytic
Case Report
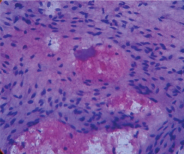
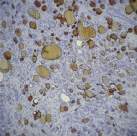
A 61-year-old male patient presented with painless thyroid swelling for 2 years duration. He was clinically euthyroid and his thyroid profile was normal. Ultrasound showed hypo echoic thyroid mass in the right lobe with cystic degeneration. FNAC [Table/Fig-1] was done in both lobes. Aspirates from the right lobe showed features of adenomatous changes and in left lobe, benign spindle cells with bland appearing nuclei. FNAC diagnosis was adenomatous hyperplasia with spindle cell proliferation and suggested thyroidectomy to rule out medullary carcinoma thyroid in view of spindle cell proliferation. Hence the patient was subjected to total thyroidectomy.
H&E (10 x)- Benign spindle cells with bland nuclei on cytological examination.
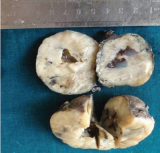
Grossly [Table/Fig-2], total thyroidectomy specimen was measuring 9x4x3cm, external surface was smooth and the cut surface showed gray white firm areas with focal cystic change, diffusely involving the entire thyroid specimen. There was no normal thyroid tissue. Tissue sections were routinely processed and stained with Haematoxylin and Eosin.
Thyroid parenchyma with gray white areas and focal cyst formation.
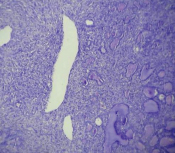
Histopathology [Table/Fig-3] sections studied from both lobes of thyroid showed spindle cell proliferation arranged in fascicles and short whorls surrounding the normal appearing thyroid follicles. The spindle cells [Table/Fig-4] were plump with elongated nuclei. Occasional lymphocytes, histiocytes and plasma cells were seen interspersed among the spindle cells. Some of the cells [Table/Fig-5] were bizarre appearing with atypical nuclei. Mitotic figures were rare. Few giant cells were seen scattered. There was no necrosis. Spindle cell neoplasm of thyroid was considered and the differentials of Medullary carcinoma thyroid/Spindle Epithelial Tumour with Thymus Like Differentiation (SETTLE) was given. Clinically there was no extra thyroidal involvement.
H&E (10x)-Spindle cell proliferations surrounding the thyroid follicles with focal cystic spaces.
H&E (40x)-Plump spindle cells having bland nuclei with lymphocytes, histiocytes and few plasma cells in the background.
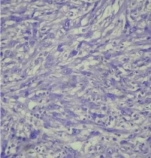
H&E (40x)-Spindle cells with indistinct cell borders, moderate cytoplasm and vesicular nuclei. Also, scattered bizarre cells showing irregular nuclei and giant cell formation.
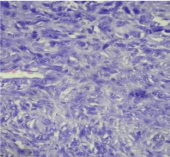
Immunohistochemistry [Table/Fig-6,7,8,9 and 10] was done with thyroglobulin, calcitonin, TTF-1, cytokeratin to rule out the possibility of spindle cell variants of papillary thyroid carcinoma, medullary carcinoma and anaplastic carcinoma, and they were negative. Vimentin [Table/Fig-6] was strongly positive; SMA [Table/Fig-9] was focally positive exhibiting myofibroblastic differentiation. CD34, S100, EMA were negative hence ruled out solitary fibrous tumour and MPNST. Anaplastic lymphoma kinase-ALK-1 [Table/Fig-7,8] was positive. Hence we diagnosed this lesion as inflammatory myofibroblastic tumour.
IHC(40x)-Cytoplasmic positivity of Vimentin in spindle cells.
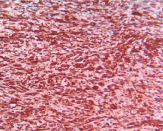
IHC (10X and 40X)- ALK-1 positivity in the cytoplasm of the spindle cells.
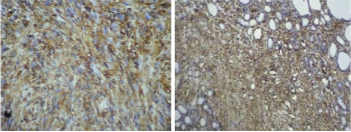
IHC(40x)-Cytoplasm SMA positivity in focal areas and in few dispersed spindle cells.
IHC(10x)- Thyroglobulin cytoplasmic positivity in the follicles and negativity in spindle cells.
The patient was doing well after one year of surgical resection of this tumour.
Discussion
Inflammatory myofibroblastic tumour is a rare spindle cell neoplasm characterized by spectrum of myofibroblastic proliferation along with varying amount of lymphoplamocytic infiltration. It was first observed and described by Brunn in1939 [1]. It was named as inflammatory myofibroblastic tumour by Umikar et al., because it was resembling a malignant tumour clinically, radiologically and histopathologically [2]. It is otherwise called as inflammatory pseudo tumour, plasma cell granuloma and plasma cell pseudotumour. Inflammatory myofibroblastic tumour commonly occurs in lung, liver and GIT.
Incidence of Inflammatory myofibroblastic tumour in thyroid is very low and only 18 cases have been reported so far in English literature [Table/Fig-11] [3–20]. There are four histological patterns described [21].
List of previously reported cases as plasmacell granulomas. # first case reported as fibrohistiocytic variant.*only three cases (including the present case) were reported as inflammatory myofibroblastic tumor fibrohistiocytic(spindle cell) variant.
| Name of the study year | Age of the patient | Clinical features | Function of thyroid | Histological pattern |
|---|
| Holck et al., (1980) [3] | 70/f | Postural dyspnoea | hypothyroid | Lymphoplasmocytic |
| Yapp et al., (1984) [4] | 61/f | Asymptomatic | hypothyroid | Lymphoplasmocytic |
| Chan et al., (1986) [5] | 35/f | Firm thyroid nodule | Euthyroid | Lymphoplasmocytic |
| Talmi et al., (1988) [6] | 51/f | Slowly enlarging painless mass | Not reported | Lymphoplasmocytic |
| De Mascard et al., (1989) [7] | 55/f | Thyroid swelling | Not reported | Lymphoplasmocytic |
| Zingrillo et al., (1995) [8] | 65/f | Asymptomatic Neck swelling | hypothyroid | Lymphoplasmocytic |
| Livoon chong et al., (2001) [9] | 29/m | Dysphagia with DM and hard mass in thyroid | Not reported | Lymphoplasmocytic |
| Martinez et al., (2002) [10] | 46/f | Nodular swelling | Euthyroid | Lymphoplasmocytic |
| Mugler et al., (2003) [11] | 46/m | Hashimoto’s Thyroiditis | hypothyroid | Lymphoplasmocytic |
| Ferrer GaArcialt et al., (2004) [12] | 41/m | Goitre | hypothyroid | Lymphoplasmocytic |
| Lauren et al., (2004) [13] | 35/f | Dysphagia with hashimoto’s | hypothyroid | Lymphoplasmocytic |
| Kriegl et al., (2007) [14] | 50/m | Dysphagia with hashimoto’s | hypothyroid | Lymphoplasmocytic |
| Fontenot et al., (2008) [15] | 58/f | Enlarging neck swelling | hypothyroid | Lymphoplasmocytic |
| Trimeche et al., (2009) [16] | 18/f | Goitre | hypothyroid | Sclerosing |
| Kojima et al., [17] (2009)# | 75/f | Painless neck swelling | euthyroid | Fibrohistiocytic |
| Barber et al., (2010) [18] | 89/f | Goitre | hypothyroid | Lymphoplasmocytic |
| Anne cremonini et al., (2012) [19] | 85/f | Goitre | | Lymphoplasmocytic |
| HyeJeongKim et al., (2014)* [20] | 50/f | Painless mass | euthyroid | Fibrohistiocytic |
| Present case* | 61/m | Goitre | euthyroid | Fibrohistiocytic |
Dominant lymphoplasmocytic pattern,
Dominant lymphohistiocytic pattern,
Prominent young and active myofibroblasts. (spindle cell or fibrohistiocytic),
Predominantly collagenized process with lymphocytic inflitrate. (sclerosing).
All of the reported cases were most commonly in women and they were all exhibiting the prominent lymphoplasmocytic pattern except for two cases one showing prominent fibrohistiocytic pattern [17]. Our case showed the third pattern with prominent young and active myofibroblasts (fibrohistiocytic).
Clinically the patients usually present with painless enlargement of thyroid, but with only dysphagia in few cases. It can be associated either with hypothyroidism or with euthyroid status. Cases have been reported with hashimoto’s thyroiditis and with goitre [12]. It can even present as diffuse lesion involving the whole thyroid or as circumscribed masses. One case have been reported as hard mass mimicking malignancy [9].
Inflammatory myofibroblastic tumour is considered as a reactive process for an underlying infection or chronic inflammation. Recent studies identified chromosomal abnormalities in pulmonary and extrapulmonary inflammatory myofibroblastic tumour supporting the neoplastic nature of this lesion.They involve the anaplastic lymphoma kinase gene located in chromosome 2p 21-24 [22].
Macroscopically they are gray white or gray tan and firm in consistency, gritty when there is calcification. It can have a diffuse involvement also, like our case.
Spindle cell pattern with young myofibroblasts is very rare in thyroid only two cases reported previously. Rest of them were lymphoplasmocyte rich and they were polyclonal in nature and these plasma cells were positive for IgG4 [23].
This spindle cell variant due to its pleomorphism and giant cells can mimic the spindle cell variant of papillary carcinoma thyroid, medullary carcinoma or anaplastic carcinoma of thyroid and SETTLE. We exclude the possibility of these malignant tumours by immunohistochemical markers – thyroglobulin, calcitonin cytokeratin, chromogranin, TTF-1 where all of these markers found to be negative. Calcifying fibrous pseudotumour and solitary fibrous tumour can have the similar morphology but it will be CD34 positive. In our case it was also negative. We also excluded the possibility of other mesenchymal tumours like MPNST, Synovial sarcoma by S100, EMA which were negative.
Inflammatory myofibroblastic tumours will be strongly positive for vimentin and focally positive for SMA in cells with myofibroblastic differentiation. Anaplastic lymphoma kinase -1 and p80 will be expressed in 36-60% of cases and they can be used in differentiating from other mesenchymal tumours [22]. ALK-1 was positive in our case.
Recent studies suggest that ALK over expression is associated with aggressive behaviour [24]. Though inflammatory myofibroblastic tumour are usually indolent, few case reports are available suggesting recurrence and malignant counterpart [25].
Conclusion
IMT has to be considered in spindle cell lesions of thyroid and clinician should be aware of recurrence and possible malignant behaviour in rare occasions. More studies are necessary to establish their neoplastic nature of this rare tumour and the therapeutic significance of ALK-.1.
[1]. Poh CF, Priddy RW, Dahlman DM, Intramandibular inflammatory myofibroblastic tumour: A true neoplasm or reactive lesion?Oral Surg Oral Med Oral Pathol Oral Radiol Endo 2005 100:460-66. [Google Scholar]
[2]. Volker HU, Scheich M, Holler S, Strobel P, Hagen R, Hermenlink HK, Differential diagnosis of laryngeal spindle cell carcinoma and inflammatory myofibroblastic tumour: Report of two cases with similar morphologyDiagn Pathol 2007 2:1-7. [Google Scholar]
[3]. Holck S, Plasma cell granuloma of the thyroidCancer 1981 48:830-32-34 [Google Scholar]
[4]. Yapp R, Linder J, Schenken JR, Karrer FW, Plasma cell granuloma of the thyroidHum Pathol 1985 16:848-50. [Google Scholar]
[5]. Chan KW, Poon GP, Choi CH, Plasma cell granuloma of the thyroidJ Clin Pathol 1986 39:1105-07. [Google Scholar]
[6]. Talmi YP, Finkelstein Y, Gal R, Zohar Y, Plasma cell granuloma of the thyroid glandHead Neck 1989 11(2):184-87. [Google Scholar]
[7]. De Mascarel A, Vergier B, Merlio JP, Goussot JF, Coindre JM, Plasma cell granuloma of the adrenal gland and the thyroid: report of two casesJ Surg Oncol 1989 41:139-42. [Google Scholar]
[8]. Zingrillo M, Tardio B, Bisceglia M, Plasma cell granuloma of the thyroid associated with Hashimoto’s thyroiditisJ Endocrinol Invest 1995 18(6):460-64. [Google Scholar]
[9]. Li voon, Chong JS, Burrows CT, Cave Bigley D, Macfarlane IA, A hard thyroid mass due to plasma cell granulomaInt Clin Pract 2001 55(5):335-36. [Google Scholar]
[10]. Martinez F, FiliParvicz E, David Hudnail S, Plasma cell granuloma of thyroid, a case report and review of literatureArch Pathol Lab Med 2002 126(5):595-98. [Google Scholar]
[11]. Mugler K, Gaido L, Ryder J, Plasma cell granuloma of thyroid, a report of rare caseEar Nose Throat J 2003 82(1):64-66. [Google Scholar]
[12]. Ferrer-Garcia JC, Costa-Talens P, Merno-Torres JF, Prieto-Rodríguez M, Vera-Sempere JF, Piñón-Sellés F, plasma cell granuloma of the thyroid and Hashimoto’s thyroiditisSouth Med J 2004 97(6):598-600. [Google Scholar]
[13]. Laurent S, Mouthon L, Longchampt E, Medical cure of plasma cell granuloma of the thyroid associated with Hashimoto’s thyroiditis: a case report and reviewJ Clin Endocrinol and Metab 2004 89(4):1534-37. [Google Scholar]
[14]. Kriegl L, Guetgemann I, Zhou H, Plasma cell granuloma of the thyroid gland mimicking carcinoma: a case report and review of the literaturePathol Res and Pract 2007 203(11):813-17. [Google Scholar]
[15]. Fontenot J, Levine SN, Adegboyega PA, Cotelingam JD, Plasma cell granuloma of the thyroid: report of case and review of literatureEndocr Pract 2008 14(5):611-17. [Google Scholar]
[16]. Trimeche M, Ziadi S, Mestiri S, Inflammatory Myofibroblastic tumour of the thyroid with sclerosing sub type-the first case reportEur Arch Otorhino Laryngol 2009 266:763-66. [Google Scholar]
[17]. Kojima M, Suzuki M, Shimizu M, Masawa N, Inflammatory Pseudotumour of the thyroid gland showing prominent fibrohistiocytic proliferation:a case reportEndocr Pathol 2009 20(3):186-90. [Google Scholar]
[18]. Barber WA, Fernando M, Chanwick DR, Plasma cell granuloma of the thyroid, a conservative approach to a rare condition and review of literatureJ Thyroid Res 2010 2010:840469 [Google Scholar]
[19]. Cremonini A, Ponzoni M, Beretta E, plasma cell granuloma of the thyroid gland, a challenging diagnostic problemInt J Surg Pathol 2012 20:500-06. [Google Scholar]
[20]. Kim HJ, Na JI, Lee JS, Inflammatory myofibroblastic tumour of the thyroid gland, a brief case reportKorean J Pathol 2014 48(4):319-22. [Google Scholar]
[21]. Margaret S, Sillo BK, Gnepp DR, Non Squamous pathology of the larynx, hypopharynx and trachea In: Gnepp DR editorDiagnostic pathology of the head and neck 4th edition newyork 2001 WB Saunders company:287-88. [Google Scholar]
[22]. Coffin CM, Patel A, Perkin S, ALK-1 and P80 expression and chromosomal rearrangements involving 2p23 in Inflammatory myofibroblastic tumourMod Pathol 2001 14:569-76. [Google Scholar]
[23]. Saab ST, Hornick JL, Fletcher CD, IgG4 plasma cells in Inflammatory myofibroblastic tumour, inflammatory marker or pathogenic link?Mod Pathol 2011 24:606-12. [Google Scholar]
[24]. Coffin CM, Hornick JK, Fletcher CDM, Inflammatory myofibroblastic tumour; comparision of clinicopathologic, histologic and immuohistochemical features including ALK expression in atypical and aggressive caseAm J Surg Pathol 2007 31:510 [Google Scholar]
[25]. Zhao HD, Wu T, Wang JQ, Zhang WD, He XL, Bao GQ, Primary Inflammatory myofibroblastic tumour of breast with rapid recurrence and metastasis:a case reportOncology letters 2013 5[1]:97-100. [Google Scholar]