Testing of Hypothesis in Equivalence and Non Inferiority Trials-A Concept
Atul Juneja1, Abha R. Aggarwal2, Tulsi Adhikari3, Arvind Pandey4
1 Scientist ‘D’, National Institute of Medical Statistics (ICMR), Ansari Nagar, New Delhi, India.
2 Scientist ‘F’, National Institute of Medical Statistics (ICMR), Ansari Nagar, New Delhi, India.
3 Scientist ‘D’, National Institute of Medical Statistics (ICMR), Ansari Nagar, New Delhi, India.
4 Scientist ‘G’ and Director, National Institute of Medical Statistics (ICMR), Ansari Nagar, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Atul Juneja, National Institute of Medical Statistics (ICMR), Ansari Nagar, New Delhi -110029, India. E-mail : atul_juneja@hotmail.com
Establishing the appropriate hypothesis is one of the important steps for carrying out the statistical tests/analysis. Its understanding is important for interpreting the results of statistical analysis. The current communication attempts to provide the concept of testing of hypothesis in non inferiority and equivalence trials, where the null hypothesis is just reverse of what is set up for conventional superiority trials. It is similarly looked for rejection for establishing the fact the researcher is intending to prove. It is important to mention that equivalence or non inferiority cannot be proved by accepting the null hypothesis of no difference. Hence, establishing the appropriate statistical hypothesis is extremely important to arrive at meaningful conclusion for the set objectives in research.
Equivalence trials, Statistical hypothesis
Introduction
Most of the research studies in heath science are dominated by observational studies because of involvement of human beings unlike other areas of science where experimental studies dominate In medical research also, the experimental studies/interventional studies need to be conducted to arrive at final cause effect relationship duly considering the design and ethical aspects [1]. The most popular interventional studies in health research are clinical trials which are conducted to evaluate any interventions such as drug efficacy, implant, devices, therapy protocols, etc [2]. A clinical trial is defined as a prospective biomedical or behavioural research study of human subjects that is designed to answer specific questions about biomedical or behavioural interventions (vaccines, drugs, treatments, devices or new ways of using known drugs treatments or devices). The outcomes of clinical trials hold good for patients, enhance therapeutic regimens and help in advanced medical practice [3]. It is important to address the designing, planning which include issues like randomisation, blinding, allocation concealment etc. It is important to mention that along with proper planning, efficient statistical analysis is equally needed that can only lead us to proper results. It has been observed that many times researcher tends to analyze the data based on conventional hypothesis of superiority trial where it was to be seen in the light of a different hypothesis leading to spurious results. The present communication attempts to discuss the issues of hypothesis with special reference to equivalence and non inferiority trials.
Establishment of Hypothesis
For any statistical analysis establishing a proper hypothesis is the basic step for correct interpretation of the data. Hypothesis is considered as a statement which has to be proved or disproved. As scientific hypothesis or statistical hypothesis is considered as the statement which can be tested based on the variable for an outcome of interest. A null hypothesis is regarded as the statement regarding the unknown values of the parameter assuming there is no association between explanatory and response variable, where as the alternate hypothesis is just contradictory to it [4]. The appropriate test statistics is used to test the hypothesis based on sample data. If researcher is interested in comparing the efficacy of two drugs then in statistical terms, the null hypothesis is defined as the situation of no difference i.e. the new drug is no better and the researcher is interested to disprove based on his scientific thinking is known as alternate hypothesis.
To make the point further, if a pharmaceutical company comes forward with a drug with intention to prove that the new drug is better than the standard drug or no treatment in practice. The null hypothesis (statistical) in this situation becomes that there is no difference between the two drugs and the alternate could be: the two drugs are different (two tailed) or: interventional drug is better or interventional drug is inferior (one tailed). Thus the establishment of the null hypothesis is extremely important to arrive at the meaningful conclusion based on analysis. The hypothesis is established before the data collection is started so as not to involve the component of bias in the study. In fact deciding about the nature of hypothesis to be tested is also required for estimation of sample size [5]. The statistical tests as a process of analysis are carried out to reject the null hypothesis in favour of alternate or accept the same. Value of test statistics is used to decide on the results favouring null or alternate hypothesis. The region in which we reject the test statistics values is known as rejection or critical region. It may be important to mention that the statistical analysis never concludes the acceptance of null or alternate hypothesis. Not been able to reject the null hypothesis does not mean that the null hypothesis is true but infers that there is not sufficient evidence against null hypothesis in favour of alternate.
Statistical Errors and Hypothesis
For analysis, the conventional null hypothesis of no difference is established to attempt for rejection which is done in the extreme circumstances i.e. test statistics falling in the critical region, an area less than 5% of the normal curve. In other words the chance that the two therapies are same is 5% or less which is nothing but type 1 error (p<0.05). Remaining 95% is termed as acceptance region (p>0.05) for the null hypothesis. The type 1 error gives us the magnitude of the chance that two treatment groups are same. If this chance is less than 0.05, the hypothesis is considered to be rejected [6]. It means that major region on which test statistics if falls does not allow to reject the null hypothesis definitely not indicating the equivalence of the therapies. Type 2 error would occur if hypothesis is not rejected but should have been rejected i.e. failing to prove superiority of interventional drug over standard when it was indeed superior. Generally the type 1 error is considered more serious than type 2 error because failing to establish superiority of interventional drug over conventional is less serious than wrongly labelling less efficient drug as superior. This cannot be taken as the rule of thumb as it depends on the situation researcher is in, considering the risk of wrongly accepting or rejecting the hypothesis.
Interpretation of the Results in Equivalence or Non-inferiority and Superiority Trial
It is important to discuss the interpretation of the results based on the results arrived at through probability. As stated above, the probability value of greater than 0.05 should not be used for establishing the equality of intervention and conventional therapy. It can only be mentioned that there is not enough evidence to disprove the null hypothesis. The chance that a hypothesis is wrongly accepted is easily understood through the concept of type 2 error which is technically mentioned as the chance of wrongly accepting the null hypothesis when it is not true.
Interestingly it is seen that null hypothesis is just reverse of what an investigator is trying to prove. It can be better understood through the [Table/Fig-1]. The superiority can also be established through studying 95% CI of the Mean difference (or odds ratio), if the confidence intervals Include 0 (or 1 in case of odds ratio) then it indicated that we have not enough evidence to say that one drug is superior to another.
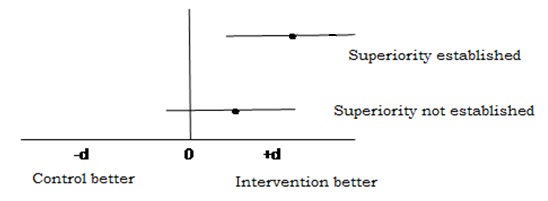
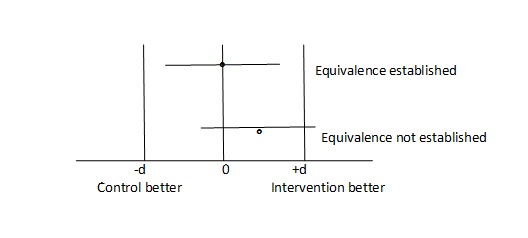
With the growing developments in medical science the efficacy of the drugs reaching at peak in efficacy the focus of the outcome of new drugs or molecule for the indications for which other drugs are being practised has shifted to other issues such as side effects, cost, convenient administration route (e.g. Intravenous to oral) with equivalent efficacy. Hence, the studies cannot be designed with conventional null hypothesis of no difference since the purpose is not to prove betterment of one drug over another [4]. The researcher tries to prove that the drug is similar but has lesser side effects or cost, involving the concept of equivalence or non-inferiority even if the drug is less efficient by a given acceptable magnitude. Just to reiterate the issue discussed above, the acceptance of conventional hypothesis of no difference cannot be termed as equivalent. Hence in this situation where the objective of the investigator is to prove equivalence/non inferiority, the null hypothesis becomes just reverse i.e. the two drugs are different and we need to reject the hypothesis of difference with a given level of precision (type 1 and II error). There is a vaccine which has a proven efficacy of 90% and is acceptable by the health delivery program. A company counters the drug in practice with a new preparation having lesser side effects, more economical and more comfortable administrative route or lesser dose with equivalent results. Considering the other factors it is considered to accept the new drug by the health system even it performs within acceptable efficacy margin (d) which could be considered as 5%. In other words the interventional drug is considered equivalent if it’s efficacy is within 85% to 95%. If this performance goes below –d of more than d then this new drug is not considered equivalent. While establishing the statistical hypothesis, the efficacy of less than 85% and more than 95% becomes null hypothesis which is attempted for rejection by two single tailed test.
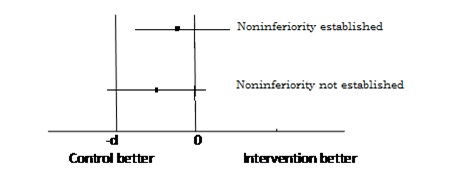
The situation what we commonly encounter, is the concern for inferiority margin i.e. the drug should not fall below –d the performance over +d which is superiority is obviously acceptable to us. Thus the researcher is concerned for non inferiority and not for equivalence. In technical terms for establishing hypothesis in non inferiority trial the region below-d becomes null hypothesis and >-d becomes alternate where as in equivalence trials the superiority part +d was also part of the null hypothesis and the region for alternate hypothesis was between –d and +d. Technically it is the situation of non inferiority, what most of the time researcher is interested in but interchangeably use with equivalence trial because of the lack of the technical concept in differentiating the nomenclature of two designs. The null hypothesis in this situation becomes, the performance of drug is less than –d and is looked for rejection through a single tailed to achieve alternate which is the performance of more than -d test unlike in equivalence trial where performance of more than +d ie superiority was also part of null hypothesis and alternate was performance between –d and +d.
Hence it is very important to understand the difference between superiority, non inferiority trial for proper designing of the study including the sample size [7,8]. [Table/Fig-2] depicts equivalence trial where as [Table/Fig-3] presents non inferiority.
While planning a clinical trial it is important to understand the design based on the objective of the study. The effect size and the equivalence of non inferiority is set by considerations of researcher and group responsible for implementation and obviously statistician who could guide through the sample size power of the study and scope for negotiating on effect size and non inferiority margins. It may further be mentioned that there is need to have a good understanding of the design of clinical trials not only to have an efficient study but is also required for the registration of the trial on clinical trial registry through a mandatory item on type of the study and sample size etc., [9].
[1]. Thatte U, Ethical issues in Clinical Research. In: Gupta SK, editorBasic Principles of Clinical Research and Methodology 2007 1st edNew DelhiJaypee Brothers:58-73. [Google Scholar]
[2]. Clinical Trials(internet). WHO (cited 21 October 2015). Available from http://www.who.int/topics/clinical_trials/en/) [Google Scholar]
[3]. Clinical Trials (internet). NIH US Department of Health and Services 2011 (cited 21 October 2015). Available from https://www.nichd.nih.gov/health/clinicalresearch/clinical-trials/Pages/about.aspx [Google Scholar]
[4]. Biau DJ, Jolles BM, Porcher R, p-Value and the Theory of Hypothesis Testing: An Explanation for New ResearchersClinical Orthopaedics and Related Research.(internet) 2010 468(3):885-92.Available from http://dx doi:10.1007/s11999-009-1164-64 [Google Scholar]
[5]. Makuch RW, Simon RM, Sample size requirements for evaluating a conservative therapyCancer Treatment Reports 1978 62:1037-40. [Google Scholar]
[6]. Juneja A, Murthy NS, Concept of and relevance of p-value in medical researchObs and Gynae Today 2003 8(6):334-35. [Google Scholar]
[7]. Blackwelder WC, Current issues in clinical equivalence trialsJournal of Dental Research 2004 83(Spec Iss C):C113-15. [Google Scholar]
[8]. Hwang IK, Morikawa T, Design issues in noninferiority/equivalence trialsDrug Information Journal 1999 33:1205-18. [Google Scholar]
[9]. Pandey A, Aggarwal A, Maulik M, Gupta J, Juneja A, Challenges in Administering a Clinical Trials Registry: Lessons from the Clinical Trials Registry-IndiaPharmaceutical Medicine 2013 27(2):83-93. [Google Scholar]