Introduction
The high blood lead level induces oxidative stress and alters the antioxidant status of battery manufacturing workers. Supplementation of vitamin C is beneficial to reduce the oxidative stress and to improve the antioxidant status of these workers.
Aim
The main aim of this study was to observe the changes in blood lead levels, oxidative stress i.e. serum lipid peroxide and antioxidant status parameters such as erythrocyte superoxide dismutase and catalase and serum nitrite after the vitamin C supplementation in battery manufacturing workers.
Materials and Methods
This study included 36 battery manufacturing workers from Western Maharashtra, India, having age between 20-60 years. All study group subjects were provided vitamin C tablets (500 mg/day for one month) and a blood sample of 10 ml each was drawn by puncturing the anterior cubital vein before and after vitamin C supplementation. The biochemical parameters were estimated by using the standard methods.
Results
Blood lead levels were not significantly altered, however, serum lipid peroxide (p<0.001, -15.56%) and serum nitrite (p<0.001, -21.37%) levels showed significant decrease and antioxidant status parameters such as erythrocyte superoxide dismutase (p<0.001, 38.02%) and catalase (p<0.001, 32.36%) revealed significant increase in battery manufacturing workers after the supplementation of vitamin C.
Conclusion
One month vitamin C supplementation in battery manufacturing workers is not beneficial to decrease the blood lead levels. However, it is helpful to reduce the lipid peroxidation and nitrite formation and enhances the erythrocytes superoxide dismutase and catalase activity.
Catalase, Erythrocyte-superoxide dismutase, Lipid peroxide, Serum nitrite
Introduction
Lead is a ubiquitous and versatile metal, which has been used by human beings over 9000 of years. But today it is a widely distributed pollutant in environment. Lead is highly resistant to corrosion, pliable, having high density, low elasticity, high thermal expansion, low melting point, easy workability, easily recycled, excellent antifriction metal and inexpensive. Due to these properties it is used in acid battery manufacturing, printing press, silver jewellery making, soldering cans, folk remedies, cable sheathing, in colour pigments, petrol additives, soldering water distribution pipes, ceramic glazes, paper industries etc, [1,2]. Lead and its compound can enter the environment at any point during mining, smelting, processing, use, recycling or disposal [1,2].
Lead present in food, beverages, soil/dust and atmospheric air is absorbed by the gastrointestinal tract and is rapidly taken up in blood and soft tissues (half-life 28-36 days) and then to bones (half-life 27 years). Lead is mainly excreted through the urine (> 90%) while lesser amounts are eliminated via the feces, sweat, hair and nails [1,2].
In unorganized lead acid battery manufacturing sectors, most of the work is accomplished manually and the workers are exposed to lead through inhalation, ingestion and dermal contact. The accumulation of lead mainly occurs in red blood cells, soft tissue such as brain, kidney & bone marrow and mineralized tissues like bone and teeth. In lead acid battery manufacturing areas lot of lead particles, dust, smoke is available, which increases the blood lead levels of the workers [3] and the variety of health issues such as dental caries [4], haematological [5,6], matrix–gamma-carboxy glutamic acid protein polymorphism [7], cardiovascular diseases [8], kidney and liver failure [9–11], oxidative stress [12,13], pteridine metabolism with neurotransmitters synthesis [14], immunological [15], genotoxicity [16], reproductive [17], neuropsychological [18], monoamine metabolites [19] have been reported in the battery manufacturing workers after the chronic exposure to lead.
Lead induces oxidative stress and alters the antioxidant status of battery manufacturing workers is well documented in several studies [12,13]. Vitamin C is strong antioxidant and it reduces oxygen-, nitrogen-, and sulfur- centered radicals [20]. Studies on lead treated rats have shown lowered levels of iron leading to impaired heme formation. However, treatment with Vitamin C restored the levels of iron [21]. Therefore, the present study was undertaken to assess the role of vitamin C on blood lead level, oxidative stress and antioxidant status of Battery Manufacture Workers of Western Maharashtra, India.
Materials and Methods
This study comprised total 36 male battery manufacturing workers from Western Maharashtra, India having the age group between 20-60 years.
Healthy, non alcoholic and non smoking male subjects were selected for the present study. Subjects who were on medication for minor and major illnesses were excluded. Before blood collection, all the study subjects were informed about the study objectives and health hazards of lead exposure and its toxicity. After obtaining written consent, demographic, occupational and clinical data was collected by using questionnaire and interview. Majority of battery manufacturing workers had complaints like loss of appetite headache, paresthesia, paresis, intermittent abdominal pain, nausea, diarrhea, constipation and myalgia. The socioeconomic status of all subjects was average. Dietary intake and food habits of all subjects were normal. The experimental protocol was approved by the institutional protocol committee and ethical clearance was obtained from institutional ethics committee. Utmost care was taken during the experimental procedure according to ICMR Guidelines 2006 [22]. 10 ml blood sample was drawn by puncturing the anticubital vein and 4 ml blood was then transferred in tube a containing heparin and same amount was also taken in plain tube. The remaining 2 ml blood was collected in EDTA bulb for biochemical parameters assays included in the study. Then all the study subjects were provided 30 tablets of 500 mg vitamin C and asked to take 1 tablet daily in the morning for 1 month and after 1 month blood samples, were again collected. Before collecting blood samples we ensured that all workers had consumed vitamin C tablets regularly at morning for one month. The biochemical parameters such as blood lead, serum lipid peroxide and nitrite, erythrocyte superoxide dismutase and catalase were estimated before and after the supplementation of vitamin C by using standard methods.
Blood lead level was estimated by using lead Care II blood lead analyser (Magellan Diagnostics, USA). The lead care II system uses an electrochemical technique called Anodic Stripping Voltammetry (ASV) to determine the amount of lead in a blood sample [23]. Lipid peroxidation was measured spectrophotometrically by the method of Kei Satoh [24]. The activity of Erythrocyte-Superoxide Dismutase (SOD) was determined by Marklund and Marklund method [25]. Erythrocyte catalase was measured by the method of Aebi [26]. Serum nitric oxide was determined by the cadmium-reduction method [27]. Statistical analysis of results was done by instant graph pad. The mean difference was considered significant at p< 0.05.
Results
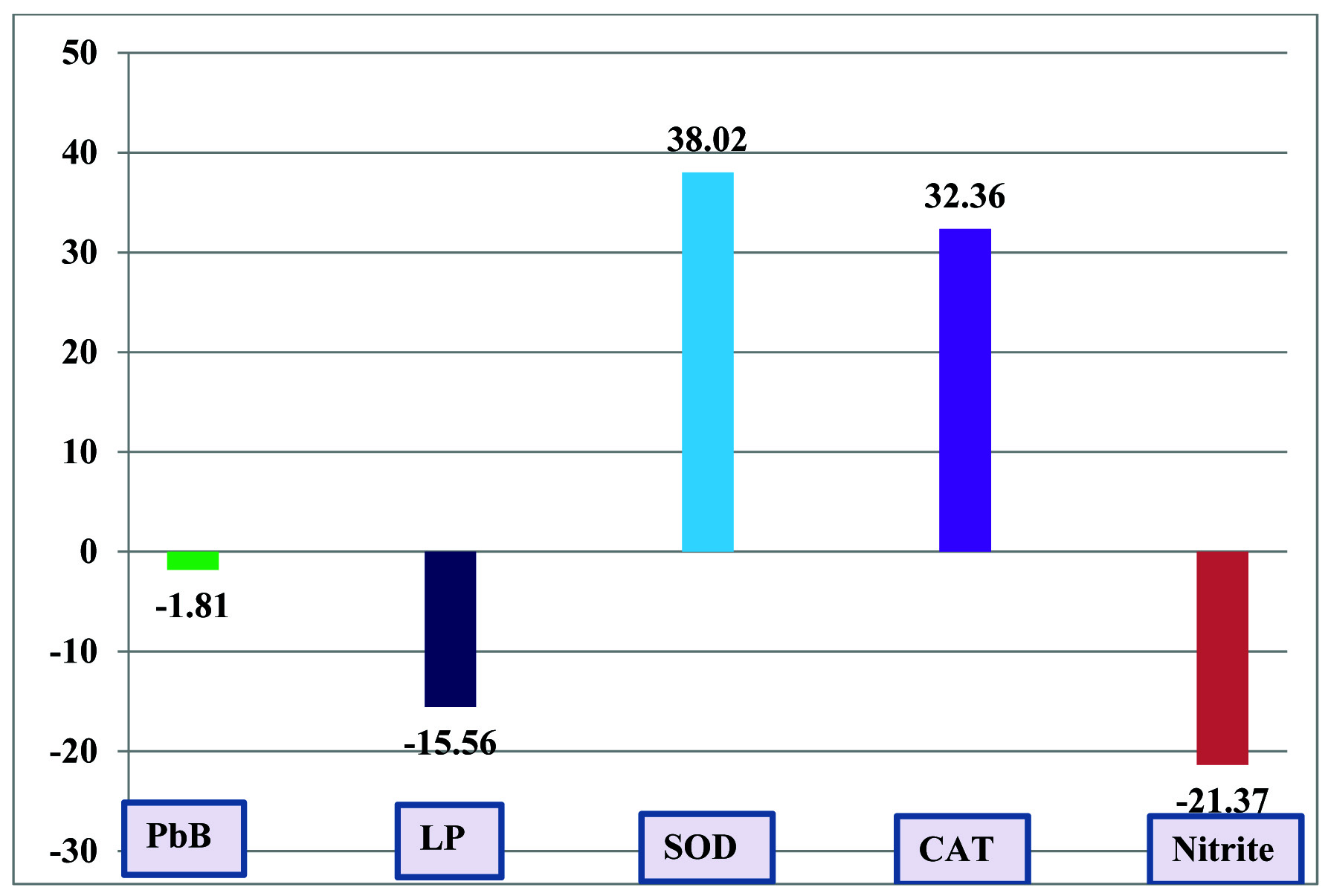
Blood lead levels were not significantly altered, however, serum lipid peroxide (p<0.001, -15.56%) and serum nitrite (p<0.001, -21.37%) levels showed significant decrease and antioxidant status parameters such as erythrocyte superoxide dismutase (p<0.001, 38.02%) and catalase (p<0.001, 32.36%) revealed significant increase in battery manufacturing workers after the supplementation of vitamin C [Table/Fig-1,2].
Blood Lead Levels, Oxidative Stress and Antioxidant Parameters of Battery Manufacturing Workers of Before and After Vitamin C (500 mg/day for 30days) Supplementation.
| Sr. No. | Biochemical parameters | Before Vitamin CSupplementation(N= 36) | After Vitamin CSupplementation(500 mg Vit. C/dayfor 30 days)(N=36) |
|---|
| A | Blood Lead Level(μg/dl) | 63.25 ± 5.39(48.7-65) | 62.10 ± 4.93·(40.6-65) |
| B | Oxidative stress parameter |
| A | Serum Lipid Peroxide(nmol/ml) | 2.12 ± 0.65(0.9 -3.2) | 1.79 ± 0.63***(0.5- 2.7) |
| C | Antioxidant Status |
| A | RBC-Superoxide Dismutase(unit/ml of haemolysate) | 5.97 ± 1.46(3.55 – 8.90) | 8.24 ± 2.10***(3.55- 11.55) |
| B | RBC- Catalase(mM/H2O2 decom/mgHb/min) | 28.70 ± 7.71(16.90- 46.48) | 37.99 ± 17.26***(16.90 – 97.18) |
| D | Serum nitrite (μmol/lit) | 51.69 ± 11.96(27.82-81.20) | 40.64 ± 8.54***(26.89-60.50) |
Figures indicate Mean ± SD values and those in parenthesis are range of values
***p<0.001 and · Non significant (Significant levels as compared to one month of vitamin C supplementation).
Percentage Change of Blood Lead Levels, Oxidative Stress and Antioxidant Status Parameters of Battery Manufacturing Workers with Respect to Before Vitamin C Supplementation
Discussion
Blood lead levels of battery manufacturing workers were not significantly altered (-1.81%) after one month vitamin C supplementation (500mg/day) indicates that the vitamin C may not be acting as chelating agent. The blood lead level of battery manufacturing workers (63.25 ± 5.39 μg/dl) was very high as compared to non lead exposed population may be due to the poor hygiene and inappropriate protection. Battery manufacturing involves the use of lead for making grid, bearing and solder. This process is usually manual and continuous release of lead into environment results into lead poisoning. Increased blood lead levels in battery manufacturing workers induces the oxidative stress and altered the antioxidant status is well documented in various studies [1,2,9,12]. Therefore, we planned to supply the vitamin C (500mg /day for one month) to all these battery manufacture workers and measured the serum lipid peroxide, nitrite and antioxidant status parameters such as erythrocyte-superoxide dismutase and catalase before and after vitamin C supplementation.
Serum lipid peroxide (p<0.001, -15.56%) and serum nitrite (p<0.001, -21.37%) levels showed significant decrease and erythrocyte superoxide dismutase (p<0.001, 38.02%) and catalase (p<0.001, 32.36%) revealed significant increase in battery manufacturing workers after one month vitamin C supplementation (500 mg/day). The lead treated rats when supplied with vitamin C showed the increased activity of catalase may be due to increased absorption of dietary iron [21]. Our study suggests that supplementation of 500 mg vitamin C daily for 1 month is sufficient enough to suppress the action of free radicals generated due to lead toxicity. Vitamin C is a water-soluble vitamin which is necessary for normal growth and development. It is required for collagen synthesis which is necessary for skin, tendons, ligaments, and blood vessels formation. Vitamin C helps in the wound healing and formation of scar tissue. It repairs and maintains cartilage, bones and teeth and acts as one of the major antioxidants [28].
Several studies have reported that the lead induces the generation of reactive oxygen species [29,30] that may be due to the interaction of lead with oxy-haemoglobin and generation of O2- - radicals in RBC and accumulation of δ- Aminolevulinic acid (δ-ALA). δ-ALA may undergo enolisation and autoxidation at pH 7.0-8.0 results in superoxide anions generation [31,32]. Accumulation of δ- aminolevulinic acid in several tissues and its increased excretion in urine is well documented in several studies [33,34]. Lead alters the RBC membrane structure and functions which may be due to its excess accumulation in them [35]. The RBC’s are more vulnerable to oxidative damage than any other cells [36,37]. Lead toxicity increases osmotic & mechanical susceptibility of RBC’s to oxidative damage [38] and alters their membrane enzymes activity [39–41] and composition of membrane proteins [42].
Increased blood lead levels in battery manufacturing workers result in the copper deficiency, which decreases the superoxide dismutase activity, since the Zn-Cu dependent nature of SOD enzyme is well documented in several studies [43–45]. Also, increased blood lead level inhibits the enzymes involved in heme synthesis and interferes in iron absorption at gastrointestinal tract resulting in decreased heme synthesis. The combined effect of these two mechanisms decreases the heme pool and catalase activity, since catalase is heme containing enzyme [1,2].
Hence, vitamin C supplementation is helpful to reduce the oxidative stress and scavenge the free radicals generated due to lead in battery manufacturing workers. Drinking a glass of lemon water in the morning can detoxify the free radicals generated in the lead exposed populations.
Limitations
The sample size of this study is small, since for estimation of one blood lead sample requires the rupees eight hundred and other biochemical parameters expenditure was different. Due to the fund constraint we have not estimated serum vitamin C level before and after supplementation of vitamin C tablets and included only selective 36 battery manufacturing workers in this study.
Conclusion
Supplementation of 500 mg/day vitamin C for 1 month in battery manufacturing workers is not sufficient to reduce the blood lead level, however it is helpful to reduce the lipid peroxide and nitrite levels and to improve the antioxidant status parameters like erythrocytes superoxide dismutase and catalase by scavenging the free radicals generated due to high blood lead levels.
Figures indicate Mean ± SD values and those in parenthesis are range of values
***p<0.001 and · Non significant (Significant levels as compared to one month of vitamin C supplementation).
[1]. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for lead, US Department of Health and Human services, Atlanta, Georgia USA: US Government Printing, 2005;102-225 [Google Scholar]
[2]. World Health Organization. Biological indices of lead exposure and body burden. In: IPCS, Inorganic lead, Environmental Health Criteria 118, Geneva, Switzerland: WHO, 1995;165:114-118 [Google Scholar]
[3]. Ibiebele DD, Air and blood lead levels in a battery factorySci Total Environ 1994 152(3):269-73. [Google Scholar]
[4]. Bamise CT, Esan TA, Ajayi JO, Olagundoye O, Oziegbe EO, Dental erosion in a road-side battery technician: case report and a review of the literatureOral Health Prev Dent 2008 6(3):249-53. [Google Scholar]
[5]. Shaik AP, Jamil K, A study on the ALAD gene polymorphisms associated with lead exposureToxicol Ind Health 2008 24(7):501-06. [Google Scholar]
[6]. Fonte R, Agosti A, Scafa F, Candura SM, Anaemia and abdominal pain due to occupational lead poisoningHaematologica 2007 92(2):e13-14. [Google Scholar]
[7]. Shaik AP, Jamil K, Polymorphisms in MGP gene and their association with lead toxicityToxicol Mech Methods 2009 19(3):209-13. [Google Scholar]
[8]. Kim KR, Lee SW, Paik NW, Choi K, Low level lead exposure among South Korean lead workers, and estimates of associated risk of cardiovascular diseasesJ Occup Environ Hyg 2008 5(6):399-416. [Google Scholar]
[9]. Patil AJ, Bhagwat VR, Patil JA, Dongre NN, Ambekar JG, Das KK, Occupational lead exposure in battery manufacturing workers, silver jewelry workers, and spray painters in western Maharashtra (India): effect on liver and kidney functionJ Basic Clin Physiol Pharmacol 2007 18(2):87-100. [Google Scholar]
[10]. Wang VS, Lee MT, Chiou JY, Guu CF, Wu CC, Wu TN, Relationship between blood lead levels and renal function in lead battery workersInt Arch Occup Environ Health 2002 75(8):569-75. [Google Scholar]
[11]. Ehrlich R, Robins T, Jordaan E, Miller S, Mbuli S, Selby P, Lead absorption and renal dysfunction in a South African battery factoryOccup Environ Med 1998 55(7):453-60. [Google Scholar]
[12]. Patil AJ, Bhagwat VR, Patil JA, Dongre NN, Ambekar JG, Jailkhani R, Das KK, Effect of lead (Pb) exposure on the activity of superoxide dismutase and catalase in battery manufacturing workers (BMW) of Western Maharashtra (India) with reference to heme biosynthesisInt J Environ Res Public Health 2006 3(4):329-37. [Google Scholar]
[13]. Gurer-Orhan H, Sabir HU, Ozgüneş H, Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workersToxicology 2004 195(2-3):147-54. [Google Scholar]
[14]. Engin AB, Tuzun D, Sahin G, Evaluation of pteridine metabolism in battery workers chronically exposed to leadHum Exp Toxicol 2006 25(7):353-59. [Google Scholar]
[15]. Queiroz ML, Perlingeiro RC, Bincoletto C, Almeida M, Cardoso MP, Dantas DC, Immunoglobulin levels and cellular immune function in lead exposed workersJournal of Environmental and Occupational Science 2012 1(1):1-5.http://www.jenvos.com [Google Scholar]
[16]. Duydu Y, Süzen HS, Aydin A, Cander O, Uysal H, Işimer A, Correlation between lead exposure indicators and sister chromatid exchange (SCE) frequencies in lymphocytes from inorganic lead exposed workersArch Environ Contam Toxicol 2001 41(2):241-46. [Google Scholar]
[17]. Robins TG, Bornman MS, Ehrlich RI, Cantrell AC, Pienaar E, Vallabh J, Semen quality and fertility of men employed in a South African lead acid battery plantAm J Ind Med 1997 32(4):369-76. [Google Scholar]
[18]. Kumar P, Husain SG, Murthy RC, Srivastava SP, Anand M, Ali MM, Neuropsychological studies on lead battery workersVet Hum Toxicol 2002 44(2):76-78. [Google Scholar]
[19]. Tang HW, Liang YX, Hu XH, Yang HG, Alterations of monoamine metabolites and neurobehavioral function in lead-exposed workersBiomed Environ Sci 1995 8(1):23-29. [Google Scholar]
[20]. Niki E, Action of ascorbic acid as a scavenger of active and stable oxygen radicalsAm J Clin Nutr 1991 54:1119S-24S. [Google Scholar]
[21]. Bhattacharjee CR, Dey S, Goswami P, Protective Role of Ascorbic Acid Against Lead Toxicity in Blood of Albino Mice as Revealed by Metal Uptake, Lipid Profiles, and Ultrastructural Features of ErythrocytesBull Environ Contam Toxicol 2003 70:1189-96. [Google Scholar]
[22]. Ethical Guidelines for Biomedical Research on Human Participants, Indian Council of Medical Research New Delhi 2006 [Google Scholar]
[23]. Magellan Diagnostics, User’s guide, Lead Care II Blood Lead Testing System.1-9. (70-6551-Lead Care II-User’s-Guide-Rev-06-USB) [Google Scholar]
[24]. Satoh K, Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric methodClin Chim Acta 1978 90(1):37-43. [Google Scholar]
[25]. Marklund S, Marklund G, modified by Nandi). Assay of Sod activity in tissueJ Biochem 1988 13:305-15. [Google Scholar]
[26]. Aebi H, Catalase methods in enzymatic analysis (ed) Bergumeryer HU 1983 vol. 3New YorkAcedemic press:276-286. [Google Scholar]
[27]. Coras NK, Wakid NW, Determination of inorganic nitrate in serum and urine by a kinetic cadmium reduction methodClin chem 1990 36(8):1440-43. [Google Scholar]
[28]. Rafi MD, Biochemical role of vitamin C. Textbook of Biochemistry for medical students2nd ed:150-51. [Google Scholar]
[29]. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M, Free radicals, metals and antioxidants in oxidative stress-induced cancerChemico-Biological Interactions 2006 160:140 [Google Scholar]
[30]. Patrick L, Lead toxicity part II - the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicityAltern Med Rev 2006 11(2):114-27. [Google Scholar]
[31]. Al-Ubaidy B, Al-Khashali DK, Numan NA, The role of oxidative stress in lead poisoningIraqi J Pharm Sci 2006 15(1):70-75. [Google Scholar]
[32]. Ahamed M, Siddiqui MKJ, Low level lead exposure and oxidative stress: current opinionsClinica Chimica Acta 2007 383:57-64. [Google Scholar]
[33]. Haeger–Arosen B, Studies on urinary excretion of 5-ALA and other haem precursors in lead workers and lead- intoxicated rabbitsScand J Clin Lab Invest 1960 12(47):1 [Google Scholar]
[34]. O’Flaherty EJ, Hammond PB, Lerner SI, Hanenson IB, Roda SMB, The renal handling of delta-aminolevulinic acid in the rat and in the humanToxicol Appl Pharmacol 1980 55:423-32. [Google Scholar]
[35]. Donaldson WE, Knowles SO, Is lead toxicosis a reflection of altered fatty acid composition of membranes?Comp Biochem Physiol 1993 104(3):377-79. [Google Scholar]
[36]. De silva PE, Determination of lead in plasma and studies on its relationship to lead in erythrocytesBr J Ind Med 1981 38(3):209-17. [Google Scholar]
[37]. Rice-Evans C, Iron-mediated oxidative stress and erythrocytes. In: Harris, J.R. (Ed.)Blood Cell Biochemistry 1990 Vol. 1New YorkPlenum Press:429-453. [Google Scholar]
[38]. Waldron HA, The anaemia of lead poisoning: a reviewBr J Ind Med 1966 23(2):83-100. [Google Scholar]
[39]. Bonting SL, Caravaggio LL, Studies on sodium-potassium-activated adenosinetriphosphatase. V. Correlation of enzyme activity with cation flux in six tissuesArch Biochem Biophys 1963 101:37-46. [Google Scholar]
[40]. Hasan J, Vihko V, Hernberg S, Deficient red cell membrane (Na+-K+)-ATPase in lead poisoningArch. Environ. Health 1971 14:313-18. [Google Scholar]
[41]. Raghavan SRV, Culver BD, Gonick HC, Erythrocyte lead-binding protein after occupational exposure. II. Influence on lead inhibition of membrane Na+, K+-adenosinetriphosphataseJournal of Toxicology and Environmental Health 1981 7(3-4):561-68. [Google Scholar]
[42]. Fukumoto K, Karai I, Horiguchi S, Effect of lead on erythrocyte membranesBr J Ind Med 1983 40(2):220-23. [Google Scholar]
[43]. Mylroie AA, Collins H, Umbles C, Kyle J, Erythrocyte superoxide dismutase activity and other parameters of copper status in rats ingesting lead acetateToxicol Appl Pharmacol 1986 82(3):512-20. [Google Scholar]
[44]. Mylroie AA, Umbles C, Kyle J, Effects of dietary copper supplementation on erythrocyte superoxide dismutase activity, ceruloplasmin and related parameters in rats ingesting lead acetate. In: Hemphill, D. D., edTrace substances in environmental health (Vol. 18) 1984 Columbia, MOUniversity of Missouri Press:497-504. [Google Scholar]
[45]. Adler AJ, Barth RH, Berlyne GM, Effect of lead on oxygen free radical metabolism: inhibition of SOD activityTrace Elem Med 1993 10:93-96. [Google Scholar]