Introduction
Type 2 Diabetes Mellitus (T2DM) accounts for 90% of global diabetic population and 9% of annual global mortality with health and socioeconomic problems [1]. Peripheral neuropathy is the most common complication of T2DM followed by cardiovascular, renal and ophthalmic complications [2,3]. Vitamin B12 (B12) deficiency can also occur in T2DM [4].
American Diabetes Association recommends metformin as the first line drug along with lifestyle modifications for T2DM [5]. Prolonged metformin use causes B12 deficiency due to malabsorption of vitamin B12 [6]. B12 deficiency may be related to eating habits as its main source is animal origin food [7]. Neuropathy of B12 deficiency is preventable than the same caused by hyperglycaemia [8]. Metformin induced B12 deficiency presenting as peripheral neuropathy may be mistaken for diabetic neuropathy [5]. B12 deficiency leads to elevated Homocysteine levels [9].
Homocysteine (Hcy) is a risk factor strongly linked to cardiovascular complications in T2DM. Hcy can be remethylated to methionine or transsulphurated to cystathionine. Former requires 5-methyltetrahydrofolate as methyl donor and vitamin B12 as a co-factor [10]. Low B12 and hyperhomocysteinaemia with normal folate status are common among vegetarian Indians [11]. T2DM complications are better reduced by controlling risk factors than the blood sugar [12]. B12 therapy without folate, effectively lowers Hcy in general population, but data are sparse in diabetics [11]. Homocysteine, which is a metabolite of vitamin B12, is used to diagnose Vitamin B12 deficiency at earlier stage [13,14].
Aim
This study was undertaken to check association of s. B12 with s. Hcy in diabetics on metformin (<5 years) also to observe whether Hcy in diabetics rises independently or not.
Materials and Methods
This study included 91 samples of enrolled participants aged 40-60 years visiting health and diabetes checkup outpatient department at Shri Krishna hospital, Karamsad. The study has been approved by the appropriate institutional ethics committee and performed in accordance with the ethical standards. Informed consent was obtained from all individual participants included in the study.
Study design
Prospective case control study in which participants enrolled according to inclusion and exclusion criteria.
Inclusion Criteria
Cases: T2DM individuals on metformin (3 months to 5 years).
Control-A: T2DM individuals not on metformin (age, sex, duration of diabetes matched).
Control-B: non-diabetics (age and sex matched).
All the cases were on metformin since diagnosis of diabetes.
Exclusion Criteria
Patients with renal or liver diseases, endocrine disorders, malabsorption syndrome, gastrectomy, non-vegetarians, Pregnant women, alcoholics, tobacco and drugs users like- Antacids, Proton Pump Inhibitors, vitamin B12 supplements (either oral or parenteral within last 2-3 years), Fibric Acid Derivatives, Thiozolidinediones, insulin were excluded.
Participants were asked to arrive between 7:30 AM and 8:30 AM after an 8–10 hour overnight fast. It was informed them in detailed regarding research work, and written consent was taken for sample collection and participation in study. The fasting blood samples were collected in plain tubes, with an aseptic blood collection technique by use of sterile gloves and thorough disinfection of vene-puncture site, through 70% ethyl alcohol. Serum was separated by centrifugation at 3000 rpm for 10 minutes within 20 minutes of blood drawn. s. B12 was measured by competitive principle of Electro Chemiluminescence Immuno Assay (Cobas e411, Roche, Mannheim, Germany) and s. Hcy by Homocysteine Enzymatic Assay for RocheTM Systems using The Diazyme Enzymatic Homocysteine (Hcy) Assay reagent (Diazyme Laboratories, USA).
s. B12 was interpreted as: >221 pmol/l (normal), 148–221 pmol/l (borderline) and <148pmol/l (deficient). s. Hcy was interpreted as: ≤15 μmol/l (normal) and >15 μmol/l (hyperhomocysteinaemia).
Statistical Analysis
Normality of data was checked by Kolmogorov-Smirnov test. s. B12 and s. Hcy mean values of groups were compared using one-way ANOVA & post-hoc tests: Tukey’s, High Significant Difference (HSD), Least Significant Difference (LSD), Scheffe’s Significant Difference (SFSD) and Dunnett two sided test. Association between s.B12 and s.Hcy checked by Pearson correlation. Results were considered significant if p<0.05 and correlation at 0.01. Statistical analysis done by SPSS (statistical package for social sciences) version.17 and Microsoft excel.
Results
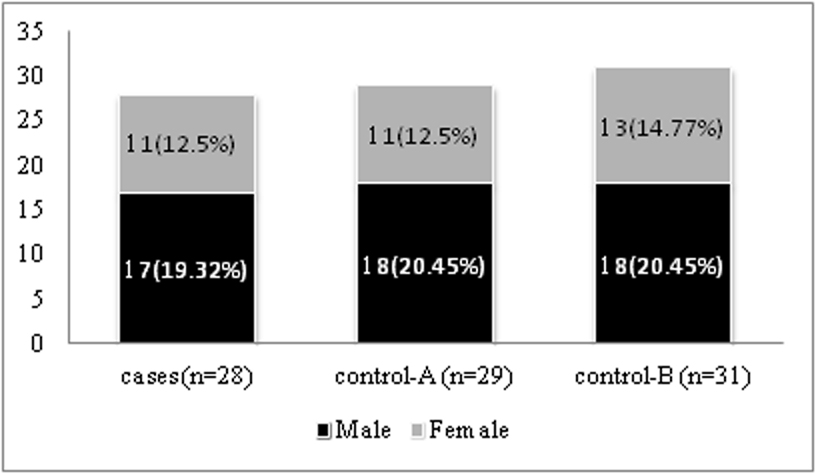
In this study mean s. B12 of total 91 subjects was 248.01 pmol/l (SD=219.13); mean s. B12 values of two cases and one control-A were outliers. Hence, 88 participants as shown in [Table/Fig-1] were taken for further analysis. Mean age in years for cases was 54.34 (SD: 5.58). Mean age in years for Control-A was 54.37 (SD: 6.1). Mean age in years for Control-B was 53.67 (SD: 5.52). Mean metformin dose was 792 mg (SD:354). Mean duration of T2DM in months for cases was 21(SD:16) and for control-A was 20 (SD:17).
Study subjects after removal of outliers.
Mean s. B12 and s. Hcy for all three groups and comparison by ANOVA shown in [Table/Fig-2]. Mean s. B12 (pmol/L) for cases, control-A and control-B were 241.93(SD: 150.61), 234.68(SD: 131.62) and 175.52 (SD: 95.23) respectively. Mean s. Hcy (μmol/L) for cases, control-A and control-B were 22.32 (SD: 10.84), 25.41(SD: 13.12), 32.07(SD: 14.22) respectively. No significant difference of mean s. B12 (p =0.090) levels in any of the pair (Case & Control-A, Case & Control-B, Control-A & control-B) while mean s. Hcy (p=0.014) differed.
Comparison by post-hoc tests for s. B12 and s. Hcy shown in [Table/Fig-3] there was no significant difference in mean s. B12 among the following pairs: cases and control-A(p>0.05) or cases and control-B(p>0.05 except LSD p=0.048) or control-A and control-B(p>0.05). Mean s. Hcy did not differ significantly (p>0.05) between cases and control-A as well as between control-A and control-B (except LSD, p=0.048). Mean s. Hcy of cases was significantly lower than that of control-B by all post-hoc tests (p<0.05). Pearson Correlation for s. B12 and s. Hcy as mentioned in [Table/Fig-4] shows strong negative correlation between s. B12 & s. Hcy in cases (r= -0.596, p=0.001) or in control-A (r=-0.6, p=0.001), control-B (r= -0.454, p =0.010) and overall (r= -0.534, p=0.000).
Comparison of mean s. B12 and s. Hcy by ANOVA.
| Variables | Cases(N*=28) | Control –A(N=29) | Control –B(N=31) | ANOVA one wayp value |
|---|
| Mean | SD# | Mean | SD | Mean | SD |
|---|
| s. B12 (pmol/l) | 241.93 | 150.61 | 234.68 | 131.62 | 175.52 | 95.23 | 0.090 (F=2.475) |
| s. Hcy (μmol/l) | 22.32 | 10.84 | 25.41 | 13.12 | 32.07 | 14.22 | 0.014 (F=4.480) |
*N- number of individuals, #SD- standard deviation
Comparison by post hoc tests among all the three groups for s. B12 and s. Hcy.
| Post hoc tests | Comparisons between groups | Dependant Variables |
|---|
| Serum B12 | Serum Homocysteine |
|---|
| p | Statistical Significance(S£ or NS#) | p | Statistical Significance(S or NS) |
|---|
| Tukey’s HSD | Cases | Control-A | 0.975 | NS | 0.637 | NS |
| Cases | Control-B | 0.117 | NS | 0.013 | S |
| Control-B | Control-A | 0.174 | NS | 0.117 | NS |
| Scheffe (SFSD) | Cases | Control-A | 0.977 | NS | 0.664 | NS |
| Cases | Control-B | 0.140 | NS | 0.018 | S |
| Control-B | Control-A | 0.203 | NS | 0.140 | NS |
| LSD | Cases | Control-A | 0.830 | NS | 0.367 | NS |
| Cases | Control-B | 0.048 | S | 0.005 | S |
| Control-B | Control-A | 0.075 | NS | 0.048 | S |
| Dunnett test* | Cases | Control-B | 0.087 | NS | 0.009 | S |
| Control-A | Control-B | 0.134 | NS | 0.087 | NS |
*In Dunnett t-tests cases and control-A was compared against control-B, #NS: no significant difference, S£: significant difference.
Pearson correlation for s. B12 and s. Hcy.
| Group | Correlation between s. B12 & s. Hcy(r*) | Pearson correlation |
|---|
| Cases | r= -0.596(p=0.001) | strong negative |
| Control-A | r= -0.6(p=0.001) | strong negative |
| Control-B | r= -0.454(p=0.010) | strong negative |
| All three groups | r= -0.534(p=0.000) | strong negative |
*r= correlation coefficient
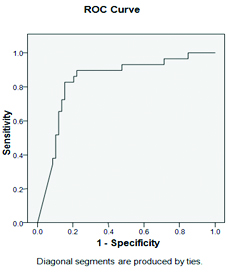
On ROC (Receiver operative characteristic) curve for the value of s. Hcy [Table/Fig-5], the area under curve value was 0.842(95% Confidence Interval:0.751 -0.934, p<0.00). At the cut off value of s. Hcy 24.62 μmol/L, sensitivity and specificity are same i.e. 82.1%. At s. Hcy 12.67 μmol/L, sensitivity of detecting B12 deficiency is 100%.
ROC Curve for the value of s. Hcy.
Based on simple regression y= a + bx equation derived. By keeping the derived values: y = 361.575-.432x (y: s. B12 value, x: s. Hcy value; 361.575: constant).
Discussion
The mean s. B12 of metformin and non metformin users were normal but nearer to the lower cut off while that of non-diabetics was in the borderline category. Most of the earlier studies’ mean B12 values were higher than this in spite of longer diabetic and/or metformin duration [9,10,15,16]. This may be attributed to non-vegetarianism, better nutrition or genetic factors than this study as Indians follow mainly vegetarian diet [11,17].
Metformin use (<5years) could not be linked with s.B12 lowering as mean s. B12 did not differ significantly between metformin users and non users which is in accordance to the study done by Shtyanberg et al., [18]. B12 deficiency is linked with increase in metformin dose and duration [5,16,19,20]. Earlier studies contrasted the current finding as they had higher mean metformin/diabetes duration and metformin doses compared to current study [3,7,10,13,15,16,20,21,22]. Even with comparable duration, dose & age; Bauman et al., and Sahin et al., contradicted this finding [23,24]. It may be due to less fluctuation in low normal s. B12 mean of vegetarian population of the current study. Here, diabetes (<5 years) could not be linked with B12 lowering as mean B12 values of diabetic and non-diabetics controls did not differ significantly, which is in contrast to the study by Reinstatler et al., [15]. No other studies reported that the diabetics on metformin had significantly higher mean B12 than non-diabetics; Reinstatler et al., contradicted the same [15]. This can be explained by frequent medical care, healthy diet and lifestyle accorded to all diabetics as compared to non-diabetics.
Metformin users with highest mean B12 value had lowest mean Hcy value and vice a versa for non-diabetics. No other study reported that the mean s. Hcy of non-diabetics significantly higher than both diabetic groups, contrasted by Reinstatler et al., [15]. Clinically, the difference was not significant as all groups had hyperhomocysteinaemia. Diabetes could not be thus linked to increase the s. Hcy level.
Metformin use less than 5 years could not be linked to rise homocysteine levels in the current study as the mean s. Hcy level of metformin users and non metformin users did not differ significantly. This finding was similar to De Jager et al., Sato et al., Ponghchaidecha M et al., Reinstatler et al., but all these studies showed normal mean Hcy and higher mean B12 values [15,16,21,25]. Shtynberg et al., indirectly showed no significant difference in mean s. Hcy levels between metformin users and non users with concurrently raised MMA [18]. This study was further supported by Wulfele et al., having no significant change in Hcy when B12 levels <310 pmol/L [10].
Strong inverse association between s. B12 & s. Hcy found in all the groups of current study has been widely seen Sato et al., stronger in metformin treated (r= -0.48, p<0.01) than the non-metformin- treated patients (r= -0.38, p=0.04); Satyanarayana et al., (r=-0.485, p=0.00) in diabetics and non-diabetics [4,21]. This study had equal correlation for both diabetic groups unlike Sato et al., [21] but stronger than that of non diabetics. Shtyanberg et al., had a rise in Hcy in accordance to MMA significantly (p<0.0003) in greater B12 deficiency prevalence group [18]. Herrmann et al., noted significant inverse correlation between s. B12 and s. Hcy with low holo Transcobalamin (holoTC) in metformin users [9] . Holo TC being costly, [26] was not estimated here. Sahin et al., De Jager et al., (p<0.0001), Wile Toth et al., (p=<.001) reported reduction in B12 with rise in Hcy following metformin therapy. Later study also showed MMA rise (p=<.001) [3,16,24]. MMA and Hcy are equally effective indicators of B12 deficiency but MMA cannot be measured in routine laboratory [27]. Moreover, metformin induced change in B12 status is not always reflected by MMA and folate [23], current study estimated only s. Hcy.
Based on the equation, hyperhomocysteinaemia was found when s. B12 remained <280.09pmol/L. This showed poor sensitivity of s. B12 in diagnosing B12 deficiency early. Obeid et al., noted that in the lower range of s. B12 (<300 pmol/L), less sensitive and specific s. B12 estimation cannot rule out B12 deficiency alone. Clinical signs of B12 deficiency can be seen with normal serum B12 levels [28]. To the best of our knowledge no other study which obtained either the equation or the ROC curve, stating the sensitivity and the specificity of s. Hcy while predicting the s. B12 value.
Limitation
Being an observational study, causal association as well as the changes from the baseline B12 and Hcy levels could not be proved. Here small group size may not give an idea about actual status of a large population. A follow up or a study involving more participants from similar population with intervention trials can overcome these limitations.
Conclusion
Strong inverse association between s. B12 and s. Hcy was found in all the three groups. Duration of less than five years in Diabetic patient or metformin use both were not linked with s. B12 lowering/s. Hcy rise as all the groups had hyperhomocysteinaemia in spite of low yet non deficient s. B12 levels. Such vegetarian populations may show greater fall in s. B12 and rise in s. Hcy in future with further diabetes and metformin continuation. Metformin is an important drug for combating hyperglycaemia, impending cardiovascular and other complications. Hyperhomocysteinaemia imposes risk of these complications. Thus if we identify and treat B12 deficiency early by measuring s. Hcy, aforementioned consequences not only among diabetics and metformin users but also among non-diabetics can be prevented or delayed. To the best of our knowledge, no other study has evaluated s. homocysteine and s. B12 relation in diabetes and metformin users of vegetarian population of Gujarat.
Funding: H. M. Patel Centre for Medical Care & Education, Charutar Aarogya Mandal, Karamsad, Gujarat, India.
*N- number of individuals, #SD- standard deviation
*In Dunnett t-tests cases and control-A was compared against control-B,
#NS: no significant difference, S
£: significant difference.
*r= correlation coefficient
[1]. Patel M, Patel IM, Patel YM, Rathi SK, A hospital-based observational study of type 2 diabetic subjects from Gujarat, IndiaJ Health Popul Nutr 2011 29(3):265-72. [Google Scholar]
[2]. Mohan V, Shah S, Saboo B, Current glycemic status and diabetes related complications among type 2 diabetes patients in India: data from the A1chieve studyJ Assoc Physicians India 2013 61(1 Suppl):12-15. [Google Scholar]
[3]. Wile DJ, Toth C, Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathyDiabetes Care 2010 33(1):156-61. [Google Scholar]
[4]. Satyanarayana A, Balakrishna N, Pitla S, Reddy PY, Mudili S, Lopamudra P, Status of B-Vitamins and Homocysteine in Diabetic Retinopathy: Association with Vitamin-B12 Deficiency and Hyperhomocysteinaemia. Malaga G, editorPLoS ONE 2011 6(11):e26747 [Google Scholar]
[5]. Ko S-H, Ko S-H, Ahn Y-B, Song K-H, Han K-D, Park Y-M, Association of vitamin B12 deficiency and metformin use in patients with type 2 diabetesJ Korean Med Sci 2014 29(7):965-72. [Google Scholar]
[6]. Mahajan R, Gupta K, Revisiting Metformin: Annual Vitamin B12 Supplementation may become Mandatory with Long-Term Metformin UseJ Young Pharm JYP 2010 2(4):428-29. [Google Scholar]
[7]. Nervo M, Lubini A, Raimundo FV, Faulhaber GAM, Leite C, Fischer LM, Vitamin B12 in metformin-treated diabetic patients: a cross-sectional study in BrazilRev Assoc Médica Bras 2011 57(1):46-49. [Google Scholar]
[8]. Aisen PS, High-Dose B Vitamin Supplementation and Cognitive Decline in Alzheimer Disease: A Randomized Controlled TrialJAMA 2008 300(15):1774 [Google Scholar]
[9]. Herrmann W, Obeid R, Schorr H, Geisel J, The usefulness of holotranscobalamin in predicting vitamin B12 status in different clinical settingsCurr Drug Metab 2005 6(1):47-53. [Google Scholar]
[10]. Wulffelé MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trialJ Intern Med 2003 254(5):455-63. [Google Scholar]
[11]. Deshmukh US, Joglekar CV, Lubree HG, Ramdas LV, Bhat DS, Naik SS, Effect of physiological doses of oral vitamin B12 on plasma homocysteine: a randomized, placebo-controlled, double-blind trial in IndiaEur J Clin Nutr 2010 64(5):495-502. [Google Scholar]
[12]. Kannel WB, Framingham Study Insights on Diabetes and Cardiovascular DiseaseClin Chem 2010 57(2):338-39. [Google Scholar]
[13]. Pflipsen MC, Oh RC, Saguil A, Seehusen DA, Seaquist D, Topolski R, The prevalence of vitamin B(12) deficiency in patients with type 2 diabetes: a cross-sectional studyJ Am Board Fam Med JABFM 2009 22(5):528-34. [Google Scholar]
[14]. Sobczynska-Malefora A, Harrington DJ, Voong K, Shearer MJ, Plasma and red cell reference intervals of 5-methyltetrahydrofolate of healthy adults in whom biochemical functional deficiencies of folate and vitamin B 12 had been excludedAdv Hematol 2014 2014:465623 [Google Scholar]
[15]. Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP Jr, Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: the National Health and Nutrition Examination Survey, 1999-2006Diabetes Care 2012 35(2):327-33. [Google Scholar]
[16]. De Jager J, Kooy A, Lehert P, Wulffele MG, van der Kolk J, Bets D, Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trialBMJ 2010 340(may19 4):c2181-81. [Google Scholar]
[17]. Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, Hyperhomocysteinaemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian IndiansAm J Clin Nutr 2001 74(2):233-41. [Google Scholar]
[18]. Shtaynberg N, Methylmalonic acid as an indicator of vitamin B12 deficiency in patients on metforminJ Diabetes Mellit 2012 02(01):72-75. [Google Scholar]
[19]. Bell DSH, Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathySouth Med J 2010 103(3):265-67. [Google Scholar]
[20]. Ting RZ-W, Szeto CC, Chan MH-M, Ma KK, Chow KM, Risk factors of vitamin B (12) deficiency in patients receiving metforminArch Intern Med 2006 166(18):1975-79. [Google Scholar]
[21]. Sato Y, Ouchi K, Funase Y, Yamauchi K, Aizawa T, Relationship between metformin use, vitamin B12 deficiency, hyperhomocysteinaemia and vascular complications in patients with type 2 diabetesEndocr J 2013 60(12):1275-80. [Google Scholar]
[22]. Tomkin GH, Hadden DR, Weaver JA, Montgomery DA, Vitamin-B12 status of patients on long-term metformin therapyBr Med J 1971 2(5763):685-87. [Google Scholar]
[23]. Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V, Increased intake of calcium reverses vitamin B12 malabsorption induced by metforminDiabetes Care 2000 23(9):1227-31. [Google Scholar]
[24]. Sahin M, Tutuncu NB, Ertugrul D, Tanaci N, Guvener ND, Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitusJ Diabetes Complications 2007 21(2):118-23. [Google Scholar]
[25]. Pongchaidecha M, Srikusalanukul V, Chattananon A, Tanjariyaporn S, Effect of metformin on plasma homocysteine, vitamin B12 and folic acid: a cross-sectional study in patients with type 2 diabetes mellitusJ Med Assoc Thail Chotmaihet Thangphaet 2004 87(7):780-87. [Google Scholar]
[26]. Nexo E, Hoffmann-Lücke E, Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utilityAm J Clin Nutr 2011 94(1):359S-65S. [Google Scholar]
[27]. Gilfix BM, Vitamin B12 and homocysteineCan Med Assoc J 2005 173(11):1360-60. [Google Scholar]
[28]. Obeid R, Schorr H, Eckert R, Herrmann W, Vitamin B12 status in the elderly as judged by available biochemical markersClin Chem 2004 50(1):238-41. [Google Scholar]