Rational use of medicine implicates that, “Patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community” [1].
The development of antacids, H2 antagonists, proton pump inhibitors (PPIs) and prostaglandin analogues has revolutionized the management of acid related upper gastrointestinal problems. The use or rather misuse and overuse of these drugs have been reported in many studies in different hospital settings [2–4]. In a study conducted in Italy by Scagliarini et al., [2], authors have concluded that acid suppressive therapy was substantially over-used in both hospital and general practice settings, mainly for ulcer prophylaxis in low-risk patients. Naunton et al., in their study for determining the appropriateness of PPIs, found that the prescribing of PPIs satisfied the approved indications, as outlined in the Australian Schedule of Pharmaceutical Benefits, in only 37.1% of cases [5].
The quality of health care, particularly as regards the rational use of drugs, depends on a wide range of factors, including a correct diagnosis, prescription of correct drugs, adequate administration for required time and considerations to cost of therapy. To cover the knowledge gap about additional aspects of gastro-protectant misuse, this study was planned to assess the prescriptions of these agents for appropriateness and rationality in a tertiary care hospital setup.
Materials and Methods
It was a cross-sectional observational study. After approval by the Institutional Ethics Committee (Ref: BVDU/MC/46 - 06/09/2012), the study was conducted in different outpatient departments (OPDs) of a tertiary care teaching hospital, Pune from August 2013 to December 2013. Written informed consent was taken from the prescribing doctors and the patients who were eligible to be included in the study. Data was collected by reviewing the prescriptions and interviewing the patients who were prescribed gastro-protectants during study period. On two week visit to each OPD, 260 prescriptions of gastro-protectants were found and analysed for their rationality.
The other details of prescribed drugs like contents, manufacturing company and prices were obtained from CIMS and Drug Today. Prescribed drugs were categorized as single drugs or Fixed Dose Combinations (FDCs) and whether they were prescribed by generic or brand name. Collected data and all these details were entered in Microsoft Excel 2013.
Statistical Analysis
For analysing the rationality aspect of the preparations prescribed- Goodman and Gillman 12th Edition (2011), Harrison’s Principles of Internal Medicine 18th Edition (2012) and American College of Gastroenterology Guidelines – 2009 were referred. For information on cost, formulation, content, manufacturing company of the prescribed drugs- CIMS and Drug Today were referred. We also estimated the cost difference between prescriptions with and without gastro-protectives in cases where they were not adequately indicated. This data was analysed by Wilcoxon signed rank test using GraphPad Prism 6.
Results
In this study, the prescribing trend of these gastro-protective agents was assessed in about 1000 prescriptions of patients attending different OPDs of the hospital. Total 260 prescriptions containing these gastro-protectives were found in which 300 such drugs were prescribed either singly or in combinations. Total 181 drugs of different brands were found prescribed, of which only 5 were generic drugs. We saw the trend that physicians prescribe fixed dose combinations as frequent as individual drugs.
Out of 260 patients, who were prescribed anti-peptic ulcer agents, 206 patients (79%) had no complaints related to acid peptic disease presently or in past. Amongst those (54 patients) who had current or past acid-peptic symptoms, only 21 patients (8%) were asked about these problems by their treating doctor. In some (13%) of the patients, presence of these symptoms was revealed by the investigator on interviewing the patient.
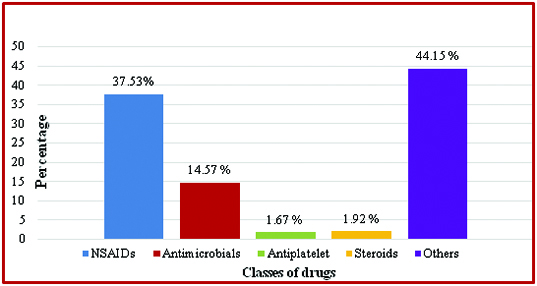
As seen in [Table/Fig-1] gastro-protective drugs were co-prescribed with different classes of drugs of which NSAIDs were the most common followed by antimicrobials. Other miscellaneous drugs found prescribed along with these gastro-protectants were multivitamins, haematinics, cough and cold remedies, enzyme preparations, etc.
Classes of drugs prescribed with gastro-protective drugs.
[Table/Fig-2] depicts that paracetamol was the most common NSAID with which gastro-protectants were co-prescribed followed by diclofenac, etodolac and aceclofenac. Of the gastro-protectants, PPIs were more prominently seen (73.77%) {Dexrabeprazole (32.7%) followed by Pantoprazole (24.6%)}. Ranitidine was prescribed in only 21.3% patients while in 2 cases, only antacids were prescribed along with NSAIDs.
Trend of co-prescription of gastro-protectives with NSAIDs.
| CHARACTERISTICS | NUMBER (%) |
|---|
| Different NSAIDs found prescribed (n=240)• Paracetamol• Diclofenac• Etodolac• Aceclofenac• Others | 114 (47.5)55 (22.9)29 (12.0)22 (9.2)20 (8.2) |
| Gastro-protectives co-prescribed with NSAIDs (n=183)• Proton pump inhibitors• H2 Blockers• Sucaralfate• Antacids | 135 (73.77)39 (21.31)3 (1.64)6 (3.28) |
| Risk factors in patients prescribed NSAIDs (n=156)• 0 Risk Factor• 1 Risk Factor• 2 Risk Factors• >2 Risk Factors | 99 (63.46)56 (35.90)1 (0.64)0 (0.00) |
Different risk factors responsible for NSAIDs induced upper gastrointestinal (GI) toxicity were also evaluated in the study population [Table/Fig-2]. These risk factors included- age more than 65 years, history of past GI event specially complicated ulcer, present GI symptoms, concomitant use of steroids, anticoagulant or anti-platelet therapy, serious co-morbidities especially cardiovascular disease, multiple NSAIDs in same prescriptions and NSAIDS in higher than recommended doses. Out of 156 patients, 99 (63%) of them had no associated risk factors for NSAID induced GI toxicity while none of the patients had more than 2 risk factors. The most common single risk factor present in this patient cohort was a prescription containing more than one NSAID.
The indications of gastro-protective drugs were rated as adequate, uncertain or no documented adequate indication for their prescription in the study. As shown in [Table/Fig-3], only 37.31% prescriptions had adequate well documented indications for these drugs in the study prescriptions which included NSAID/Low Dose Aspirin (LDA) use in high risk patients and presenting symptoms of acid peptic disease. In 62.69% prescriptions the indications were uncertain or had no documented reason (co-prescribed with single NSAID, antimicrobial agent, allergy, with coxibs in no risk patients, etc) were found for gastro-protective agents. We could not find any reason for the prescribed gastro-protectant in 39 prescriptions given for complaints like- nausea and vomiting, weakness and disturbed sleep, decreased oral intake, chest pain, constipation and co-prescription with miscellaneous drugs (Enzyme preparations, Multivitamins, Benzodiazepine, Ayurvedic formulation, Fibrates, Montelukast, Levocetrizine, Antithyroid drugs, Beta blocker, Laxative, Estadiol). [Table/Fig-4] shows the number of prescriptions in which there was a possibility of drug interactions amongst the gastro-protective drugs prescribed. There were 19 prescriptions in which an antacid preparation was co-prescribed along with either a PPI or H2 blocker. The anti-peptic ulcer drugs were also seen co-prescribed with antiplatelet agents, vitamin B12 and calcium preparations.
Rating of indications and results of use of gastro-protective agents.
| INDICATIONS | PRESCRIPTIONS (%)n=260 |
|---|
| Adequate documented indications | 97 (37.31) |
| 1. NSAIDs / Low Dose Aspirin in high risk group | 77 (29.62) |
| 2. Acid peptic disease symptoms | 20 (7.69) |
| Uncertain / No documented indications | 163 (62.69) |
| 1. NSAIDs in no risk patients (no reason) | 63 (24.23) |
| 2. Antimicrobials (no reason) | 33 (12.69) |
| 3. NSAIDs with Antimicrobials (no reason) | 19 (7.30) |
| 4. Corticosteroids in no risk patients (no reason) | 5 (1.92) |
| 5. Coxibs alone (no reason) | 2 (0.77) |
| 6. Allergy (uncertain) | 2 (0.77) |
| 7. Others (no reason) | 39 (15) |
Anticipated drug interactions with the prescribed gastro-protectants.
| Anticipated drug-drug interactions | No. of Prescriptions |
|---|
| Antacids + Proton Pump Inhibitor | 15 |
| Antacids + H2 blocker | 4 |
| Proton Pump Inhibitor + Sucralfate | 5 |
| Clopidogrel + Esomeprazole | 2 |
| Vit B12 + gastro-protective agent | 24 |
| Calcium + gastro-protective agent | 20 |
| Aspirin + H2blocker / PPI | 6 |
| Ciprofloxacin + Antacids | 1 |
[Table/Fig-5] shows that out of 260 prescriptions, 68 prescriptions (26%) were erroneous with total 75 different pharmaceutical errors. Formulation error and strength error were counted when there was either a wrong mention or no mention of them in the prescription. Some of the errors co-existed in the prescriptions.
Types of errors in the study prescriptions.

In our study, we found that PPIs were advised twice in 88 (28%) prescriptions or even thrice a day in one prescription or with no dosing time mentioned in prescriptions (>50%). The dosing instruction of PPIs in relation to meals or with co-prescribed antacids was lacking in majority of the prescriptions (98.33% and 100% respectively).
We drew the average cost of all the prescriptions in cases where the gastro-protectant drug had either uncertain or no indication. This cost was then compared with the price of same prescriptions calculated without the gastro-protectant drug and the difference was found statistically significant (p<0.0001) [Table/Fig-6].
Comparison between cost of prescriptions with and without gastro-protectants in un-justified cases.
| Parameter | With gastro-protectants | Without gastro-protectants | p-value |
|---|
| Average Cost of prescription | Rs.57.96 + 50.83 | Rs.44.12 + 45.95 | < 0.0001 |
Values are expressed as Mean ± SD. Comparison done using Wilcoxon signed rank test
Discussion
According to the WHO’s definition of rational drug use, the first objective is to consider patient’s need for that particular drug. The major indication for gastro-protective drugs in the current clinical scenario is prophylaxis or treatment with co-prescribed ulcerogenic drugs. We have evaluated different drugs co-prescribed with gastro-protectives in all our study prescriptions. In this study, it was found that gastro-protectives were predominantly co-prescribed with four major classes of drugs- NSAIDs, antimicrobials, corticosteroids and anti-platelet drugs for the purpose of gastro-protection [Table/Fig-1]. Similar results were obtained in a study by Vipin Kumar Singh et al., in gastroenterology department of a tertiary care teaching hospital in North India, where also NSAIDs were the most common group of drugs co-prescribed with gastro-protectives [6]. In a study by Niklasson et al., in Sweden, corticosteroids were predominantly seen co-prescribed with gastro-protectives in hospitalised patients with pulmonary disease [7].
Various guidelines and literature from textbooks specify the indications of gastro-protectants for symptomatic relief in acid-peptic diseases/symptoms or prophylaxis with NSAIDs / low dose aspirin against upper gastrointestinal damage in high risk group. In our study, total 156 patients with NSAIDs were co-prescribed one or two gastro-protectants. Risk factors which were evaluated for NSAID-related GI complications included a previous GI event, especially if complicated, age, and concomitant use of anticoagulants, corticosteroids, other NSAIDs including low-dose aspirin, high-dose NSAID therapy, and chronic debilitating disorders, especially cardiovascular disease. It was found that amongst these patients, 99 patients (63.46%) had no risk factors and hence no indication for gastro-protection. A total of 56 (35.90%) patients had only 1 risk factor and 1 (0.64%) patient had 2 risk factors and hence use of gastro-protectant is justified if given in a correct dose.
The gastro-protective agents seen prominently co-prescribed with NSAIDs in our study were PPIs, Dexrabeprazole and Pantoprazole. Ranitidine was the only H2 blocker prescribed in standard dose in 21.3% patients on NSAIDs for GI prophylaxis. In a study conducted by Ajay Kumar et al., in an orthopaedics outpatients department of a tertiary care hospital in West Bengal, Famotidine (46.12%) was the most frequently used gastro-protective co-administered with non-steroidal anti-inflammatory drugs [8]. Standard doses of H2 blockers should not be used for the prevention of NSAID related upper GI toxicity, since they are ineffective at preventing NSAID related gastric ulcers. Double doses of H2 blockers and standard doses of PPIs are effective prophylactic agents based on the results of endoscopic studies [9,10]. A strategy of a COX-2 inhibitor with a PPI offers the greatest GI safety [11]. In the present study, we encountered the prescription of Etoricoxib (COX-2 inhibitor) in 2 cases but it was given with ranitidine and in patients with no risk factors.
We found that adequate documented indications for gastro-protectives were present only in 97 (37.3%) patients. Out of these, 20 patients were prescribed gastro-protectives for treatment of acid-peptic symptoms (there was no patient with endoscopically diagnosed peptic ulcer, GERD, ZES, GI bleeding or with diagnosed H.pylori infections in the study cohort). In the remaining 77 patients, these drugs were possibly given for prophylaxis in high risk NSAIDs users.
Drug interactions are another concern with acid suppression. By altering gastric pH, gastro-protectants may affect the pharmacokinetics of number of drugs. Such a possibility was there when these gastro-protectants were co-prescribed with Aspirin, Vitamin B12 and Calcium supplements. Thus it generally is prudent to avoid concurrent administration of antacids and drugs intended for systemic absorption. Most interactions can be avoided by taking antacids 2 hours before or after ingestion of other drugs [12]. Antacids may alter the rate of absorption and subsequent bioavailability of the H2 receptor antagonists. In present study, in 4 prescriptions antacids were co-prescribed with H2 blockers without any instructions mentioning a gap should be there between these two drugs’ administration. Even for PPI, an acidic pH in the parietal cell and canaliculi is required for drug activation and thus PPI should not be taken at the same time with antacids. In present study, 15 patients were prescribed antacids and PPI with no written instructions of avoiding same time administration of both the drugs.
There are many CYP450-related interactions between PPIs and other drugs. The potential interaction between PPIs and clopidogrel has attracted attention because of possible serious adverse effects. An FDA safety alert in November 2009 [13] recommended avoiding the use of omeprazole or esomeprazole with clopidogrel, warning that the CYP-mediated interaction could reduce clopidogrel’s effectiveness. Because of insufficient data, the FDA made no specific recommendations concerning other PPIs. In two patients in this study, esomeprazole should have been avoided for same and other PPI should have been prescribed along with clopidogrel.
Illegible handwriting in the prescription may be source of fatal consequences and a leading cause of medication error. The prescribing errors could be broadly classified into two types-errors in decision making and errors in prescription writing. A survey from Italy [14] had revealed that 1 in 4 prescriptions were not fully completed or were illegible; overall 23.9% of prescriptions were illegible and 29.9% of prescriptions were incomplete. Even advanced nations like UK [15] had reported 15% of the prescription to be containing one or more errors in critical care units. A study of prescriptions dispensed to elderly patients at a primary health care centre in Mexico [16] also found high potential prescription error (53% of total prescriptions). Most of the prescription errors were due to omissions of dosage, administration route and length of treatment and may potentially cause harm to the elderly outpatients.
Homology in the drug names from different classes may create serious issues. In the present study, 9 spelling errors in drug names were found. Strength of the drug was not mentioned in many prescriptions and amongst them 11 drugs was available in market in more than one strength under same brand name. It was also found that, in 55 (21%) prescriptions, formulation error was present. Frequent such errors which we came across were mention of tablets in place of capsules and vice a versa. A study done by Vaishali et al., at a rural tertiary care setup has shown that, there was lack of the mention of drug strength in more than 25% of OPD prescriptions [17]. A recent study by Stasiak P et al., has shown that even in the emergency care setup, prescriptions with mention of wrong dose (28 of 99, 28.3%), incomplete prescription (27 of 99, 27.3%) and wrong frequency of drug dosing (15 of 99, 15.2%) are found [18]. Such lacunae in prescriptions might lead to inappropriate dispensing of the drug by pharmacists and serious consequences.
PPI are recommended to be taken as a single daily dose. Our findings also suggest that dose and dosing schedule of gastro-protectants are common medication errors in the prescriptions. This is evident from the results that 89 patients out of 206 who were advised PPIs had this type of dosing errors in their prescriptions. In more than half of the prescriptions, time of dosing of gastro-protectives was not mentioned. It is proven that patients receiving twice-daily PPI therapy were likely to have more co-morbid conditions and greater health care utilization and overall costs compared with patients using once-daily PPI therapy. Some facts regarding gastro-protectants, which patients are not aware of but should be instructed properly include -
Absorption of H2 blockers are decreased by antacids.
PPI should be taken before breakfast in the morning.
Sucralfate should be taken on an empty stomach 1 hour before meals (as it is activated by acids).
Use of antacids within 30 minutes of a dose of sucralfate should be avoided.
To avoid most drug interactions, antacids should be taken 2 hours before or after ingestion of any other drugs.
In the present study, it was found that gastro-protectants were prescribed for uncertain or no documented indications in 163 (62.69%) prescriptions. Hence we calculated the cost burden of unnecessary gastro-protectants in these 163 prescriptions. Our results show that if these un-indicated gastro-protective drugs were not prescribed, the daily cost of the prescription would be significantly (p<0.0001) reduced. Thus this fashion of unnecessary gastro-protectant drug use should be curtailed by stringent policies of rational drug prescribing and clinicians should be trained and regularly evaluated for following these policies.
Conclusion
Clinicians prescribe gastro-protectants without evaluating the need for them and hence patients may face the dire consequences of irrational use of these drugs. Rational drug prescribing policy must be implemented in all clinical settings to curb the misuse of gastro-protectants.
Values are expressed as Mean ± SD. Comparison done using Wilcoxon signed rank test