It is well established that plaque is an initiating factor for the development of gingivitis when it comes in contact with periodontal tissue [1]. Gingivitis is a chronic inflammatory disease of gingiva that is characteristized by no attachment or alveolar bone loss and affects more than 90% of world’s population [1].
Gingivitis if not treated can lead to the destruction of periodontal apparatus resulting in periodontitis [2]. This suggests that the prevention of periodontal diseases must be based on measures directed at supragingival plaque control. This prevents gingivitis [3] and is a primary requisite for good oral hygiene [4]. Toothbrushes (manual or electric), floss, wood sticks and interdental brushes are various mechanical means used to remove supragingival plaque [5]. Despite the availability of numerous oral hygiene tools, even the most motivated person will not always be able to completely remove all the plaque [6]. The recognized inadequacies in the mechanical plaque control practices of many individuals have fuelled a year-long search for chemical agents to control plaque [7].
A large number of commercial chemical plaque control agents are available, but none without shortcomings. Chlorhexidine is considered as a gold standard [8], but is not a magic-bullet [9] and cannot be prescribed for extended periods as it may cause tooth staining, taste disturbance and in rare cases, painful desquamation of the oral mucosa [10]. Besides chlorhexidine rinses, only essential oils have been extensively used as mouthwashes. However, the alcohol content and its unpleasant taste are unacceptable to some patients and their religious beliefs [7]. Now-a-days, patients are more concerned about their oral health and side-effects of artificial chemical products. Thus, in this world of growing connection between oral health and herbal medicine, the natural occurring substances in herbs offer a gentle and enduring way of restoration of health in most valuable and least detrimental way.
Herbal medicine, a therapeutic and preventive approach, treats various diseases by plants and their extracts. Herbal medicine is both promotive and preventive in its approach [11]. It is a comprehensive system, which uses various remedies derived from plants and their extracts to treat disorders and to maintain good health There is evidence of successful treatment in literature of various oral diseases like bleeding gums, halitosis, mouth ulcers and tooth decay by using herbs like Triphala [12], tulsi patra [13], green tea [14], neem [15], clove oil [16], pudina [16], aloe vera [17]. Punica Granatum or pomegranate belongs to the Punicaceae family and is a shrub native to Asia. The peel and arils are a major source of bioactive compounds such as polyphenols, flavonoids, tannins, minerals and vitamins [18] and have been used as an astringent, hemostatic agent, and as a drug for diabetic control [19]. The most abundant polyphenols in pomegranate juice are hydrolyzable tannins called punicalagins [20], which have been shown to have free radical scavenging properties in laboratory experiments and may have dietary value as antioxidants [21]. Eating pomegranate could place anti-bacterial and antioxidant agents into the mouth and gingival areas. Mouthwash and toothpaste can be used as a carrier for daily exposure to these active agents. Therefore, the present study was designed to evaluate and compare invitro, the antimicrobial effect of pomegranate- containing, herbal and chlorhexidine mouthwashes on reference strains of Streptococcus mutans (S.mutans), Streptococcus salivarius (S.salivarius) and Aggregatibacter actinomycetemcomitans (A.a), by determination of Minimum Inhibitory Concentration (MIC).
Thus, the aim of the study is to evaluate the antimicrobial activity of pomegranate-containing mouthwash in eliminating oral biofilm forming bacteria.
Materials and Methods
The research protocol was approved by Ethical Committee and Review Board of Pandit Deendayal Upadhyay Dental College, Solapur, Maharashtra, India. It was conducted on three different organisms and three different types of mouthwash. The mouthwashes were divided into three groups:
Group A: 0.12% Chlorhexidine mouthwash (Hexidine by ICPA).
Group B: Herbal Mouthwash (Hiora mouthwash, The Himalaya Drug Company, Bangalore, India).
Group C: Pomegranate Mouthwash (Life-extension, USA). Chlorhexidine was considered as control and other two as test groups. These mouthwashes were used at a concentration of 75ml, 50ml, 25ml, 10ml and five ml per 100 ml. Reference strains of S.mutans (ATCC 25175), S.salivarius (ATCC 7073), and A.a (NCTC 9710) (Hi-Media Laboratories Pvt. Ltd, India) were selected as being colonizers in dental biofilm formation. Organisms were grown on prefabricated blood agar plates (Hi-Media Laboratories Pvt. Ltd, India). Pure growth suspensions of the respective organisms were prepared (Department of Microbiology Nathojirao G Halgekar Institute of Dental Sciences (Maratha Mandal), Belgaum, India. Blood agar plates were prepared for diffusion and the bacteria were lawned on the plates. Plates were dried and 5 wells of approximately 6mm in diameter were cut with the help of cork. Mouthwashes were added in three plates in 5 different concentrations. Plates were incubated in a CO2 jar for 24 hours at 37°C. Inhibition zone diameters were measured. The smallest range of product dilution preventing bacterial growth with the formation of inhibition zones was considered as MIC and was measured with a digital caliper in millimeters. All tests were performed in duplicate.
Statistical Analysis
ANOVA test was done to compare the dilution of mouthwashes for a particular organism and Tukey’s multiple comparison tests were done to find the exact difference. Multivariate analysis of variance (ANOVA) was done to compare the dilution of mouthwashes for a particular organism and Tukey’s multiple comparison tests were done to find the exact difference.
Results
Standard values for disc diffusion are, 26mm for S.mutans, 30mm for A.a and 26mm for S.salivarius [Table/Fig-1]. Chlorhexidine (0.12 %) presented zone of inhibitions (ZOI) between 38.46% - 96.15% for all the three organisms; while Hiora presented a ZOI ranging from 33.33% - 69.23% but was resistant at <10 ml of dilution. Pomegranate mouthwash presented ZOI ranging from 38.48 to 57.69%, but resistant at <10ml and for S.mutans, and at <25 ml for A.a and S.salivarius.
Percentages of zone of inhibitions of the three types of mouthwash at different concentrations for the three organisms.
| Samples | 75ml | 50ml | 25ml | 10ml | 5ml |
|---|
| S. mutans | Group A | 83.46% | 76.42% | 69.23% | 46.13% | 38.46% |
| Group B | 50% | 46.15% | 38.46% | - | - |
| Group C | 57.69% | 50% | 38.48% | - | - |
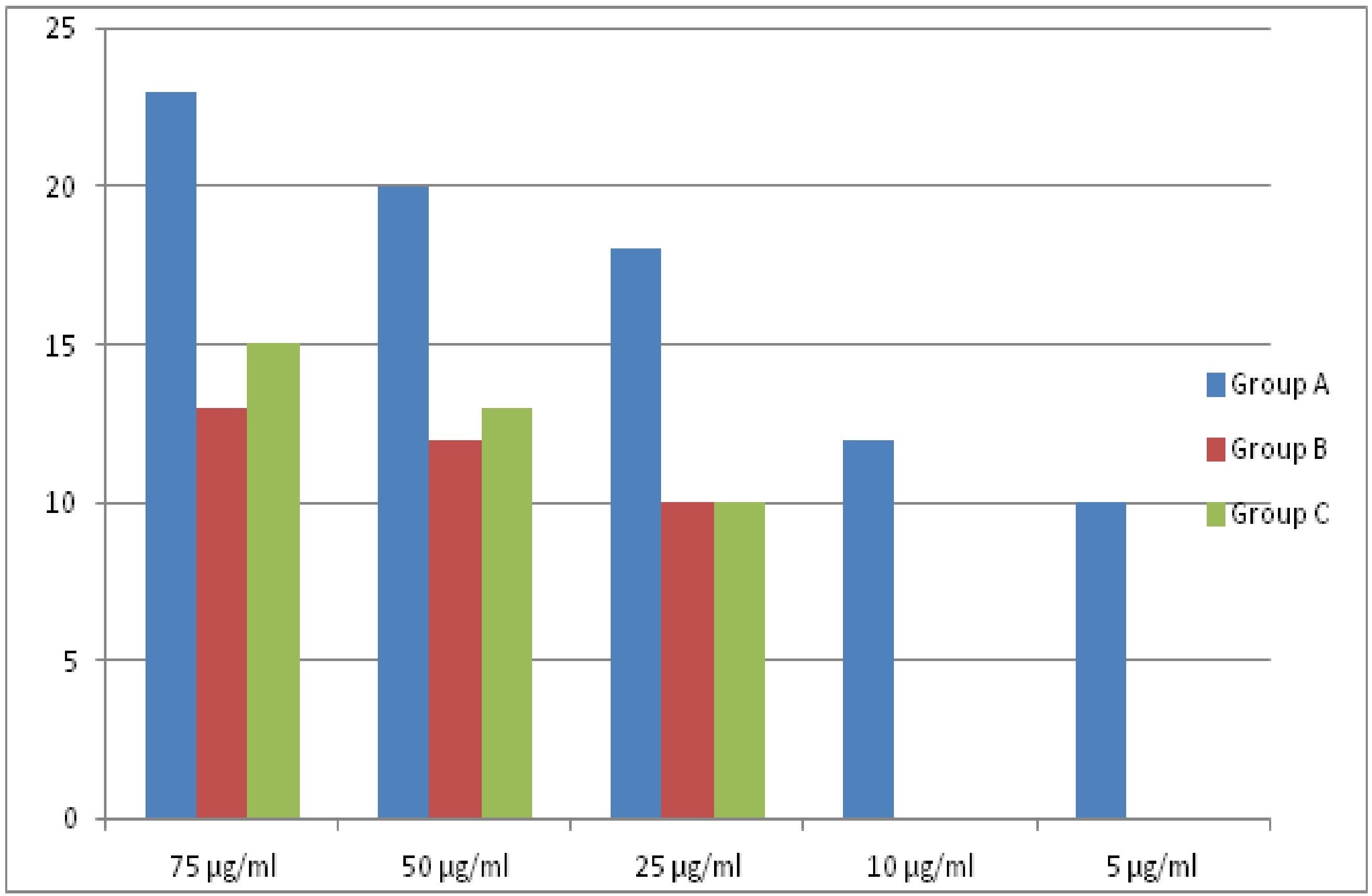
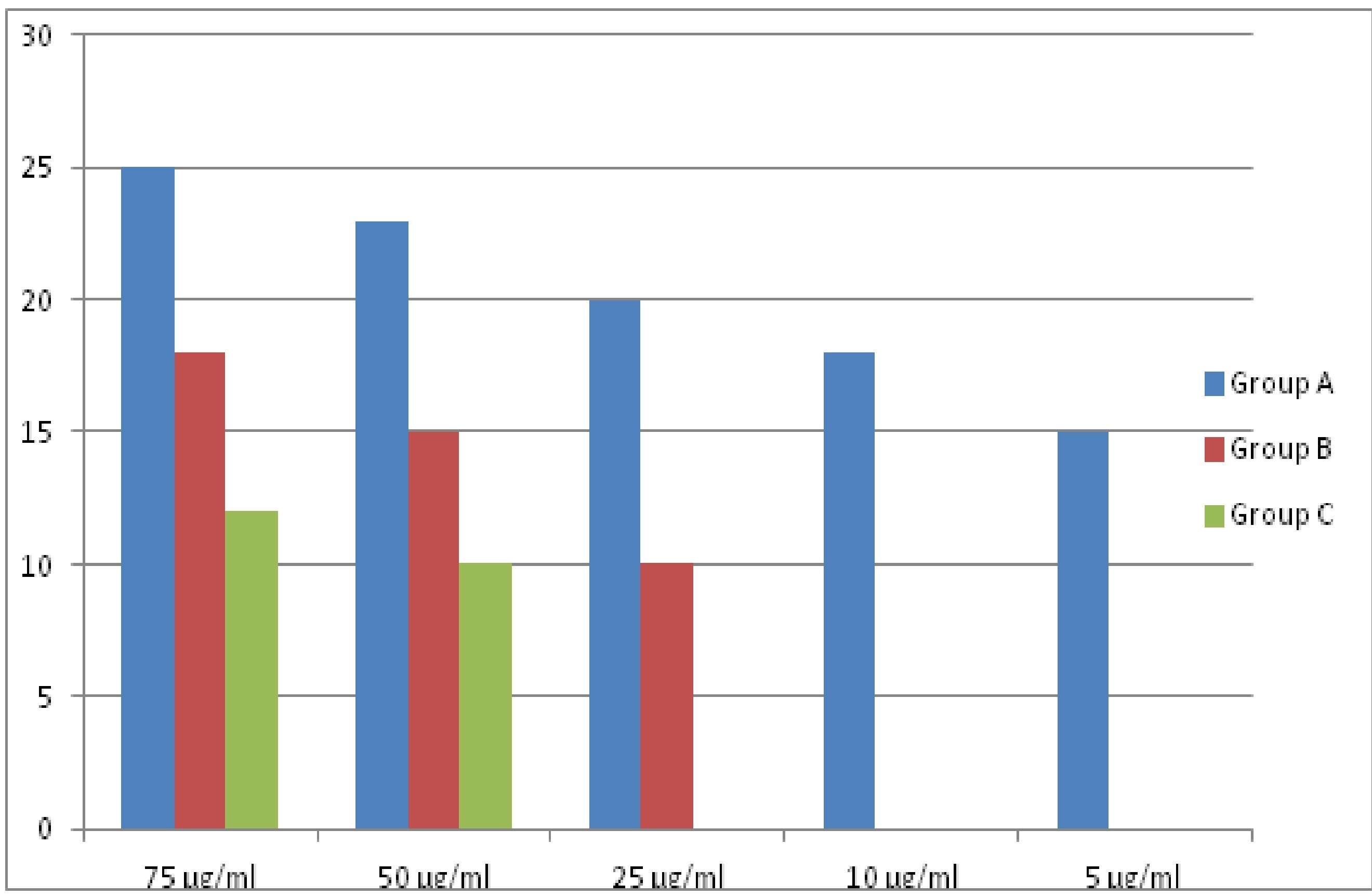
| S. salivarius | Group A | 96.15% | 88.46% | 76.92% | 69.23% | 57.69% |
| Group B | 69.23% | 57.69% | 38.46% | - | - |
| Group C | 46.15% | 38.46% | - | - | - |
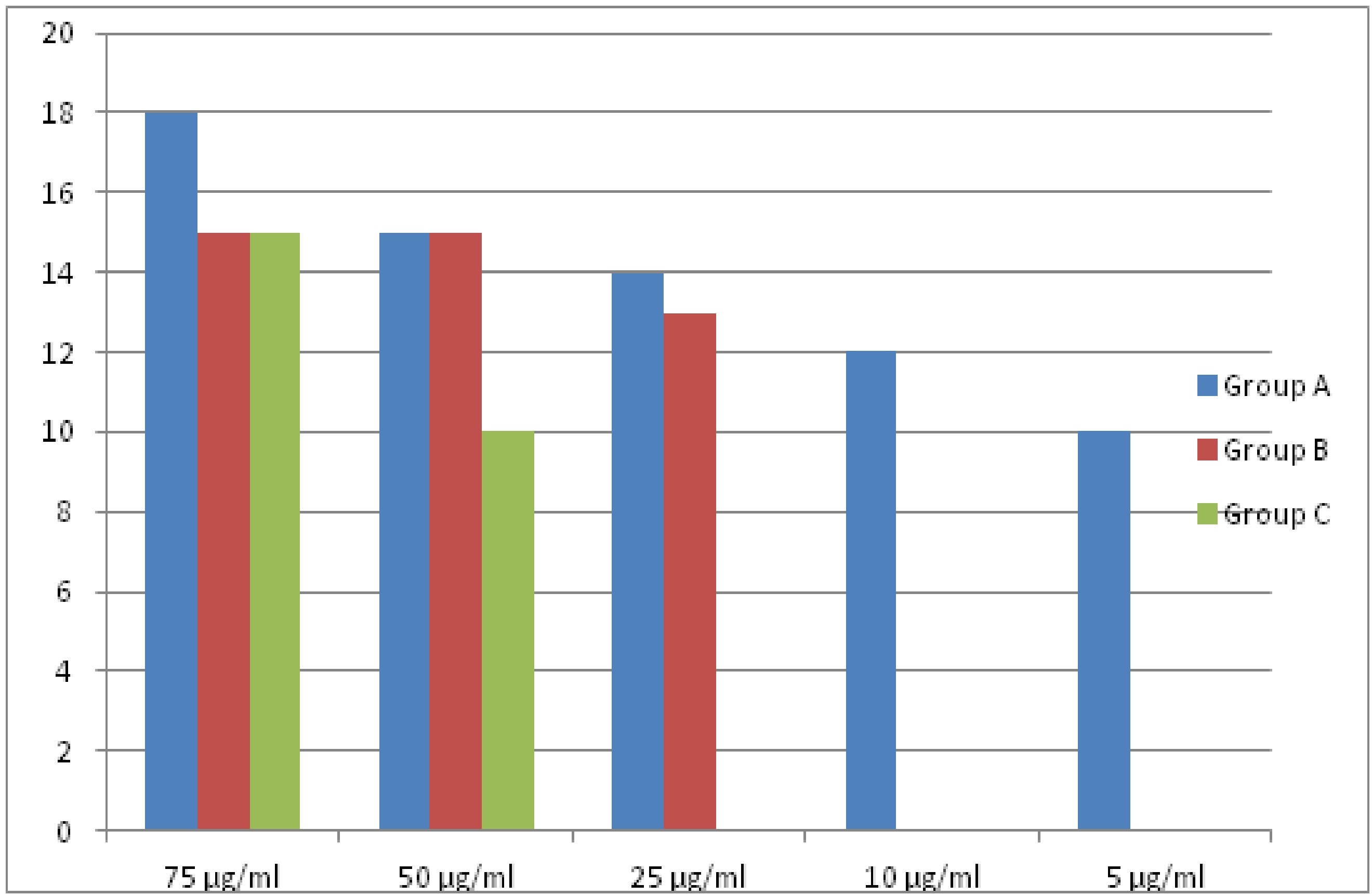
| A.a | Group A | 60% | 50% | 46.67% | 40% | 33.33% |
| Group B | 50% | 50% | 43.33% | | - |
| Group C | 50% | 33.33% | - | - | - |
S.mutans was more susceptible to chlorhexidine at all dilutions tested in the study but showed resistance to pomegranate and herbal mouthwash at a concentration <25ml. A.a and S.salivarius were susceptible to chlorhexidine at all dilutions tested but showed resistance to herbal at <10ml and to pomegranate at <25ml. [Table/Fig-2] shows the exact difference between different groups of mouthwashes for S.mutans. Group A was significantly more effective than Group B and C at higher dilutions i.e. 75, 50 and 25 ml. But no statistical difference was found between Group B and C. Significant difference was seen between all the three Groups at 50ml and 75 ml of dilution. At 75 ml concentration, a statistical difference was found between Groups B & C and groups A & B; and at 50 ml between Groups A &C. [Table/Fig-3,4 and 5] are the bar diagrams showing a comparison between three types of mouthwash.
Tukey’s multiple comparison tests for S. mutans, S.salivarius, and A.a. *Statistically significant at p 0.05 level
| (I) Groups | (J) Groups | Mean Diff (I-J) | Std. Error | p-value | 5ml |
|---|
| S. mutans | 75 ml | Group A | Group B | 10.00000* | .81650 | 0.000* |
| Group C | 8.00000* | .81650 | 0.000* |
| Group B | Group C | -2.00000 | .81650 | 0.109 |
| 50 ml | Group A | Group B | 8.00000* | .81650 | 0.000* |
| Group C | 7.00000* | .81650 | 0.000* |
| Group B | Group C | -1.00000 | .81650 | 0.483 |
| 25 ml | Group A | Group B | 8.00000* | .81650 | 0.000* |
| Group C | 8.00000* | .81650 | 0.000* |
| Group B | Group C | .00000 | .81650 | 1.000 |
| S.salivarius | 75 ml | Group A | Group B | 7.00000* | .81650 | 0.000* |
| Group C | 13.00000* | .81650 | 0.000* |
| Group B | Group C | 6.00000* | .81650 | 0.001* |
| 50 ml | Group A | Group B | 8.00000* | .81650 | 0.000* |
| Group C | 13.00000* | .81650 | 0.000* |
| Group B | Group C | 5.00000* | .81650 | 0.002* |
| A.a | 75 ml | Group A | Group B | 3.00000* | .81650 | 0.024* |
| Group C | 3.00000* | .81650 | 0.024* |
| Group B | Group C | 0.00000 | .81650 | 1.000 |
| 50 ml | Group A | Group B | 0.00000 | .81650 | 1.000 |
| Group C | 5.00000* | .81650 | 0.002* |
| Group B | Group C | 0.00000 | .81650 | 1.000 |
Bar diagram showing difference between three mouthwash at different concentration for Streptococcus mutans.
Bar diagram showing difference between three mouthwashes at different concentration for Streptococcus salivarius.
Bar diagram showing difference between three types of mouthwash at different concentration for Aggregatibacter actinomycetemcomitans.
Discussion
In a quest to find an ideal agent for plaque control medicinal plants have emerged as another possibility since they have been used successfully in alternative medicine.
To the best of our knowledge in the current literature, there are very few conparative studies referring to the antimicrobial action of pomegranate, herbal and chlorhexidine mouthwashes against oral biofilm forming organisms. Therefore, the present study was designed to evaluate and compare the antimicrobial effect of chlorhexidine, herbal and pomegranate-containing mouthwashes against S.salivarius, S.mutans and A. actinomycetemcomitans Recent studies have reported that untreated patients with periodontitis have high recovery rates of S.mutans from saliva, tongue dorsum, buccal mucosa and supra and subgingival plaque [22]. Periodontally treated patients show higher rates of root caries, even over 80%, which is higher in subjects with high counts of S. mutans. It has been isolated more frequently from root caries than from non-carious root tissues and it is believed to participate in the aetiology of the lesions [23–25].
Relative to other oral and non-oral microorganisms, S. salivarius exhibits a tendency to adhere to the oral epithelial cell and has a tendency to populate epithelial sites in vivo [26]. It constitutes a high percentage of the total facultative streptococci in samples from the tongue and cheek mucosa of adults [27,28] A. actinomycetemcomitans being a secondary colonizer does not initially colonize clean tooth surface but adheres to the bacteria already in the plaque mass [29]. It is considered as a key pathogen because it is strongly associated with periodontal disease, progression and unsuccessful therapy [30].
The present study demonstrated inhibitory action against S. mutans and S. salivarius, which are primary colonizers and A. actinomycetemcomitans, a secondary colonizer. Chlorhexidine was capable of preventing the growth of all three organisms, even at 5ml of concentration. Hiora became ineffective at <25ml of concentration. Pomegranate containing mouthwash became ineffective at <25ml of concentration for S.salivarius and A.actinomycetemcomitans and at <10ml of concentration for S.mutans. However, minimal difference in the inhibition zones was observed with pomegranate and hiora mouthwashes.
Chlorhexidine is counted as the least harmful and most effective antimicrobial agents for reduction of plaque [31], gingivitis, and S. mutans levels [32]. Findings of the present study are in accordance with other similar studies in the literature with respect to the antimicrobial activity of chlorhexidine [32,33].
In the present study, chlorhexidine was used as a control for comparison of inhibition zones. It was seen to be the effective for S.salivarius with a zone of inhibition ranging from 96.15% to 57.69% (25-15mm). For S.mutans zone of inhibition ranged from 83.4% to 38.4% (23-10mm) and for A.actinomycetemcomitans 60% to 33.33% (18-10mm) at all the tested concentrations. MIC of chlorhexidine was 5 ml for the tested organisms (zone of inhibition was seen till 5ml of concentration) [Table/Fig-1].
The absence of any adverse effect is the biggest advantage of natural herbs barring this, all herbal mouth rinses are alcohol/sugar-free, which are found in most ‘over the counter’ products. Thus, by use of herbal mouth rinses, the use of these ingredients can be avoided, which is a step forward in achieving better oral hygiene and thereby better health [9]. These naturals herbs act as antimicrobials, which not only control the disordered growth of oral microbiota, but also overcome the hazards caused by conventional antimicrobial resistant species [34]. They demonstrated antibacterial action because most of these herbs contain flavonoids, which inhibit the enzymatic activity and also act on cells, distrupting the cytoplasmic membrane [35].
Hiora mouthwash is a herbal preparation, made from natural herbs with their beneficial properties like anticariogenic and antiplaque (due to S. persica which contains trimethylamine, salvadorine, chlorides, high amounts of fluoride and silica, sulphur, vitamin C, small amounts of tannins, saponins, flavonoids and sterols) [36] antibiotic (due to the presence of Piper betle and Elettaria cardamomum) and anti-inflammatory and immunity booster effects (due to the presence of Terminalia Billerica) and natural flavorings agents (Mentha and Trachyspermum ammi) [37].
Nagavalli (piper betle) present in Hiora mouthwash claims to inhibit Streptococcus and Actinomyces species and its aqueous extract shows plaque inhibitory action [34], Pilu (Salvadora persica) minimizes the plaque formation, possibly due to its antimicrobial activity against S. aureus, S.pyogens, Lactobacillus species [36]. In the present study, herbal mouthwash (Hiora) demonstrated zone of inhibition ranging from 50-38.46% (13-10mm) for S.mutans; 69.23-38.46% (18-10mm) for S.salivarius and 50 - 43.33% (15-13mm) for A.a., which are less than the zone of inhibitions produced by chlorhexidine (Group A); for all the three organisms. MIC for Hiora mouthwash was seen at 25ml of concentration for all the three organisms.
Another study [38] investigating the antimicrobial activity of Listerine (Peridex) and herbal mouth rinse with 0.12% chlorhexidine gluconate against oral micro-organisms like S. sanguis, S. mutans and A. viscosus, reported that herbal mouth rinse was more effective, producing the largest zone of inhibition against the three tested bacteria as compared to Listerine. However, in comparison with peridex the zone of microbial inhibition produced by the herbal mouth rinse was larger against S. sanguis and S. mutans and similar against A.viscosus, which doesn’t fall in line with our study. Mehta S in their study has shown that the tested herbal mouthwash had a better antimicrobial effect on S.mutans than chlorhexidine which also contradicts our results [39]. However, the contents of herbal mouthwash used in above studies were not displayed. Therefore exact comparison with the present study is not possible.
Pomegranate containing mouthwash showed a zone of inhibitions ranging from 57.49-38.48% (15-10mm) for S.mutans; up to 25 ml of concentration (no inhibition was seen below this concentration); whereas for S.salivarius, the zone of inhibition was seen from 46.15-38.4% (12-10mm) and for A. actinomycetemcomitans 50-33.33% (12-10mm). For both the organisms, no inhibition was seen below 50 ml of concentration. These zones of inhibitions are of lesser diameters at all dilution points when compared to chlorhexidine and also than group B for two of the organisms tested except S.mutans.
According to Lee et al., 2005 pomegranate extracts inhibits sucrose digesting enzyme and the organisms responsible for plaque formation by competitive and non-competitive inhibition [40]. Polyphenolic flavonoids present in pomegranate are effective in maintaining good oral health thereby eliminating the development of gingivitis [40]. Menezes et al. studied the effect of hydroalcoholic pomegranate extract and found it to be effective against microorganism in the dental plaque, by reducing the CFU/ml by 84% [19], suggesting it as an effective treatment alternative against the bacteria causing dental plaque [19]. Badria and Zidan in 2004 reported that antibacterial action of pomegranate flavonoids was moderate against strains which are relevant to gingivitis [41].
A study conducted by Bhadbhade et al., showed that A. actinomycetemcomitans was most resistant to pomegranate mouthwash and was inhibited at 62.5 ml (similar results were seen in our study) and become ineffective at 50% concentration [7]. The results of our study are also consistent with the study conducted by Vahabi S on S.mutans which showed that punica granatum and S. persica which is one of the components of Hiora mouthwash, had the most antibacterial activity. At 100% w/v, Punica granatum had strong antibacterial activity against S.mutans, and its antibacterial activities were significantly less than Chlorhexidine and more than Miswak (S.perisca) which is in favor with our article [42].
Limitation
Although the results are not as good as chlorhexidine, pomegranate, and herbal mouthwashes have shown inhibition against the tested organisms. The inconsistencies in our study can be explained on the basis of short incubation i.e. 24 hours. Studies with longer incubation periods are warranted. Results obtained from invitro study cannot be directly extended to clinical situation; but, they do provide reproducible and dependable means for testing and comparing ‘the antimicrobial activity of various mouthwashes. More studies using longer duration can be performed. Even for invivo studies, parallel or crossover models can be considered in further research.
Conclusion
All the three types of mouthwash demonstrated antibacterial activity against the three biofilm forming organisms tested but at variable concentrations. Although chlorhexidine still continues to be the gold standard, pomegranate-containing or herbal mouthwash can be easily substituted for long term use, avoiding the side effects of chlorhexidine.
Ethical Approval: Ethical approval is obtained from Institute Ethical Committee and Review Boards.
Conflict of Interest: I confirm that the manuscript is original and has not been published elsewhere. Nor is it sent to any other journals for consideration. The manuscript in its submitted form has been read and approved by all authors. There is no financial relationship between any author and any commercial firm(s), which may pose a potential, perceived, or real conflict of interest.