Tuberculosis is one of the most important public health problems worldwide and is declared as “global emergency” by the World Health Organization [1]. An estimated nine million fell ill with tuberculosis in 2014 and 1.5 million died worldwide [2].
Detecting patients with active Pulmonary Tuberculosis (PT) disease is an important component of tuberculosis control programs, as early appropriate treatment renders these patients noninfectious and interrupts the chain of transmission of tuberculosis. Under the program conditions diagnosis of PT is based on sputum examination. Acid Fast Bacilli (AFB) staining by fluorescent microscopy remains a fundamental tool of diagnosis, but may be negative in up to 50% of active pulmonary tuberculosis [3]. Alternative methods for acquiring sputum in these smear negative cases include – Induced Sputum (IS), Bronchial Washings (BW), Broncho-Alveolar Lavage (BAL) and Gastric Washings (GW) samples. Each method has its own pros and cons and diagnostic yield varies accordingly.
Bronchoscopy is an invasive procedure, just accessible in the large health care sectors, needs specialists for execution, expensive and may not be possible if the substantial quantities of patients are to be tried. Induced sputum strategy is a simple, easy to perform, noninvasive, cost effective alternative method [4].
The present study aims to compare the results of sputum induction and bronchial washings in diagnosis of smear-negative pulmonary tuberculosis.
Materials and Methods
One hundred and thirty patients of suspected pulmonary TB with negative sputum smear during the period of one year from August 2014 to July 2015 in pulmonology department, Katuri Medical College Hospital were enrolled in the study. The inclusion criteria were: 1) patients with respiratory symptoms suspicious of pulmonary Koch’s like cough for 2 weeks or more, evening rise of temperature, dyspnoea, loss of weight and appetite; 2) sputum either not produced or sputum negative for AFB on two samples; 3) chest radiograph showing changes consistent with active pulmonary TB; and 4) age more than 18 years. Patients with uncontrolled asthma or chronic obstructive pulmonary disease, active haemoptysis, unstable angina and patients already on anti-tubercular therapy were excluded from the study. Informed consent was taken from all the patients and the study was approved by Institutional Ethics Committee.
For all the 130 selected patients a detailed clinical history, thorough clinical examination was made and necessary investigations like chest radiography, sputum induction, and flexible bronchoscopy were done. Computed Tomogram (CT) of chest and mantoux skin test was done wherever needed. Each patient’s chest radiographs and CT chest were evaluated independently by two senior chest physicians. Abnormalities compatible with possible active disease such as cavitary lesions, consolidation, patchy fluffy shadows, infiltrates and miliary pattern were noted.
Sputum induction was done by using 10 ml of 3% hypertonic saline through ultrasonic nebulizer (Beuer Ultrasonic Nebulizer IH50) according to Ireda et al., protocol [5]. Inhalation continued till the patient had either produced good amount of sputum or 15min time elapsed. Sample was collected in a sterile container, concentrated by modified Petroff”s method and examined for AFB by fluorescent staining technique in a designated microscopy centre. The procedure was done in a well ventilated room with adequate safety precautions for the staff members who supervised the procedure. The patient was closely monitored during the procedure and 1hour post procedure. The nebulizer equipment was decontaminated with 2 % glutaraldehyde.
All the above patients underwent flexible bronchoscopy (Karl Storz) after six hours fasting on the same day trans-nasally under local anaesthesia with 2% lignocaine. Bronchial washing was performed by instilling 20 ml normal saline at room temperature through the working channel of the fibreoptic bronchoscope and aspirated into a suction trap. The sample processing was done as described above. After the procedure was over, the patient was observed for development of any pneumothorax, haemorrhage, infection and cardiac arrhythmias for 24-48 hours. Bronchoscope was thoroughly cleaned with water and sterilized by immersing in 2% gluteraldehyde for one hour.
All the patients were initiated on anti-tubercular treatment under Revised National Tuberculosis Control Program (RNTCP) and were followed up at monthly intervals in the initial intensive phase and at bimonthly intervals in continuation phase. The clinical and radiological response was evaluated. Of the total 130 patients, during follow up period six patients failed to turn up and an alternative non-tuberculosis diagnosis was made in four patients. These ten members were excluded in the final study group and results were statistically analysed.
Results
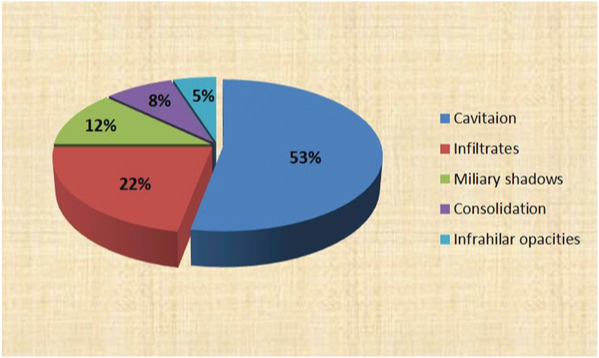
Out of 120 patients in the study group, 78 were males and 42 were females. Most patients were in the age group of 31-50 years (62.5%) with a mean age of 38 years [Table/Fig-1]. The main symptomatology of patients was cough (95%), expectoration (88%), fever (80%) and loss of appetite (75%). Radiologically, an exclusive upper zone involvement was seen in 70% of patients (n=84) while 25% of other patients (n=30) had multi-lobar involvement including upper zones and six patients had exclusive lower zone involvement. Most common findings on chest X-ray are cavitation in 53% cases (n=64) followed by fluffy or nodular infiltrations in 22% patients (n=26), miliary pattern in 12% (n=14), consolidation (8.3%, n=10) and infrahilar distribution (5%, n=6) [Table/Fig-2].
Clinico-epidemiological profile of smear negative pulmonary tuberculosis patients.
| Character | Sub set | Total number (=n) | Percentage (%) |
|---|
| Gender | Male | 78 | 65 |
| Female | 42 | 35 |
| Age | 11-20 y | 9 | 7.5 |
| 21-30 y | 24 | 20 |
| 31-40 y | 42 | 35 |
| 41-50 y | 33 | 27.5 |
| 51-60 y | 12 | 10 |
| Symptoms | Cough | 114 | 95 |
| Expectoration | 106 | 88 |
| Fever | 96 | 80 |
| Loss of appetite | 90 | 75 |
| Dyspnoea | 75 | 63 |
| Chest pain | 38 | 32 |
| Haemoptysis | 18 | 15 |
| Zonalinvolvement | Upper zone | 84 | 70 |
| Multi lobar | 30 | 25 |
| Lower zone | 6 | 5 |
Radiological pattern of smear negative tuberculosis.
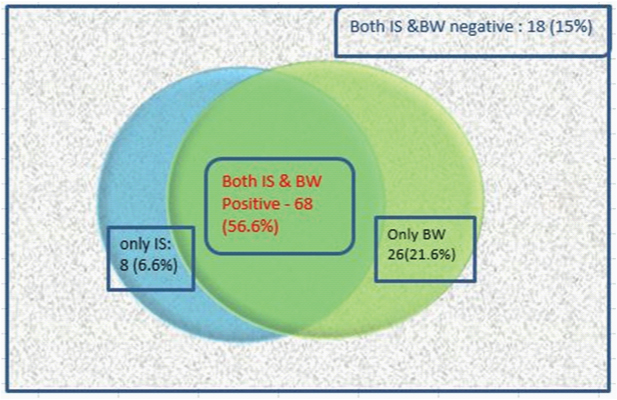
Of the total 120 patients, induced sputum smear examination for acid fast bacilli was positive in 76 cases (63.3%) and bronchial washings detected AFB in 94 cases (78.3%). Neither of the methods detected AFB in 18 patients (15%). Sputum induction procedure additionally detected AFB in 8 cases where bronchial washings results were negative (6.6%). Bronchoscopy yielded extra positivity of 26 cases in induced sputum negative scenario (21.6%) [Table/Fig-3]. There appears a fair agreement between the two procedures (kappa coefficient is 0.337).
Diagnostic yield of induced sputum vs bronchial washings in SNPTB.
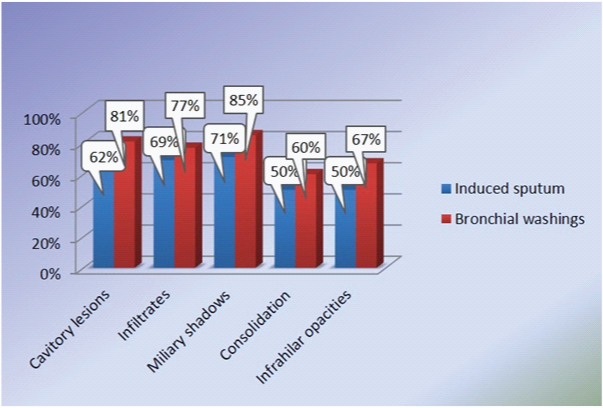
In pulmonary cavitary lesions, sputum induction detected bacilli in 62% (40 out of 64cases) and, bronchoscopy guided washings detected in 81% cases (52 out of 64 cases).The sensitivity rate in fluffy, infiltrative lesions was 69 % & 77% for SI and BW successfully. Miliary pattern nodules have a positivity rate of 71% in SI and 86% in BW [Table/Fig-4].
Comparison of induced sputum with bronchial washings in different radiological manifestations.
Discussion
As a destroyer of mankind, tuberculosis has no equivalent. It continues to intimidate the human race since time immemorial. Diagnosis of active pulmonary tuberculosis is an important component of TB control programs in all countries, as early institution of anti-tuberculosis treatment makes the patients less infectious and interrupts chain of transmission of TB. Although sputum microscopy is the most appropriate, low cost, highly specific investigation to diagnose pulmonary tuberculosis, in 22-61% of the cases, sputum smear negative and culture positive status is observed [6]. Causes of smear negativity include low bacterial load (less than 10,000 bacilli/ml), poor quality of sputum sample (submitting saliva as sputum), improper preparation & examination of smears, people with late stage HIV disease, immune suppressed patients and children [7].
Sputum smear-negative pulmonary tuberculosis (SSN-PTB) still remains a common problem faced by the clinicians. Over 50% of smear-negative patients would be needing chemotherapy by 12 months if left untreated [8]. The mortality rate for smear-negative, culture-positive cases was 14.1% compared with the 34.7% observed in smear-positive patients [9]. Thus, early diagnosis of active smear negative disease is also important.
Various methods have been used for the diagnosis of disease activity in smear negative cases. They are sputum induction, gastric lavage, trans thoracic needle aspiration, bronchial washings, broncho alveolar lavage, bronchial biopsy and newer molecular methods [10].
Sputum induction procedure was first used by Hensler et al., for the diagnosis of active pulmonary Koch’s disease [11]. The ultrasonic nebulizer delivers a fog of hypertonic saline that moves the interstial fluid into lower airways by osmotic gradient and repeated acts of coughing by the patient aids in expectoration [12]. Commonly used agents are hypertonic saline of varying concentrations viz, 3%, 4.5%, 5% glucose and salbutamol [13]. Induced sputum is also helpful in diagnosis of pneuomocystis pneumonia [14], eosinophilic asthma [15], pleural tuberculosis [16], interstial lung disease [17] and lung cancer [18].
In the present study, induced sputum smear positivity of 63.3% is higher, compared to previous studies by L.Saglam et al., with 47% positivity and Hartung et al., study with 42% positivity [19,20]. In another large study of 129 subjects by T Mc Williams et al., induced sputum AFB sensitivity was found to be very high, 96% [21]. The higher positive results may be related to the difference in the methodology as each patient went through three induced sputum procedures and also because both fluorochrome and Ziehl Neelsen staining methods were used for AFB detection. William et al., concluded that since chances of missing diagnosis with single induced sputum procedure are high, three induced sputum tests should replace FOB in investigating a case of TB suspect [21].
Fibreoptic bronchoscope (FOB) was first invented by Dr Shigeto Ikeda in 1968. The advantage of fibreoptic bronchoscopy over the rigid bronchoscopy is that, the procedure is done under local anaesthesia and a wide range of visibility upto subsegmental bronchi. Bronchscopy now-a-days has wider availability and provides excellent sample for the diagnosis of suspected cases of tuberculosis.
In the present study, bronchial washings smear results were positive in 78.5% of cases. In previous studies by Charoenratanakul et al., Fujii et al., and Chawla et al., positive BW/BL results ranged from 25-72% [22–24]. Some of the studies also included post bronchoscopy sputum and cultures, which made the result increase upto 90% [25,26]. The wide range of differences in the results may be related to differences in the procedure, co-operation of patients, experience of bronchoscopist, and quantity of fluid for irrigation and dose of xylocaine used.
The chest radiographic appearances of the study subjects were evaluated and it is observed that induced sputum smear positivity (70%, n=18/26) and bronchial washings smear positivity (77%, n=20/26) were higher in patients presenting with fluffy nodular infiltrates followed by cavitary lesions (62% & 81% for IS and BW). In the present study, 10 patients presented with consolidation pattern, five cases were AFB positive by induced sputum (50%) and six were by bronchial washings (60%). In six cases of infra hilar TB, induced sputum was positive in 50% (3/6) and bronchial washings positive in 66% (4/6). In two studies by Chan et al., Jain et al., where lower lung field TB cases were evaluated, bronchial smear positivity was observed in 75% & 67% of the cases respectively [26,27].
All the study subjects were initiated on ATT and they were followed up regularly at monthly intervals in intensive phase and 2 monthly intervals in continuation phase. The 18 cases which were negative for AFB by both induced sputum and bronchial washings showed good clinical and radiological response on starting anti-tuberculosis treatment. Rest of the cases positive for AFB either by induced sputum or bronchial washings or both responded well to ATT. The study is limited by lack of culture facilities for Mycobacterium tuberculosis.
Though this study shows that bronchial washings smear positivity is higher compared to induced sputum for detecting active tuberculosis, sputum induction is a good alternative for flexible bronchoscopy. Although in developed countries with good resource settings, FOB is the initial procedure of choice for smear negative PTB, lately there is a renewed interest in sputum induction procedures, due to the advent of HIV & AIDS pandemic. Sputum induction procedure has several advantages, as it is cost effective, safe, well tolerated, has low risk of nosocomial TB transmission, more available and doesn’t need expertise. Conde et al., observed that there is no significant difference in the yield of AFB smear or cultures obtained by sputum induction or bronchial lavage as induced sputum has a diagnosticyield in agreement with the results of FOB for diagnosis of tuberculosis in both HIV seronegative and seropositive patients [28]. On the other hand, FOB is a semi-invasive procedure, time consuming, expensive, requires trained personnel and special equipment and not widely available in developing countries and resource poor settings. Hence, it is suggested that the best course of action in a patient with suspected sputum smear negative pulmonary TB would be, sputum induction with hypertonic saline as the initial procedure of choice, which can be repeated twice / thrice in a day or two consecutive days. If the patient still remains induced sputum smear negative and if the clinical likelihood of tuberculosis is high, starting ATT and closely monitoring patient and reserving bronchoscopy to those patients who do not improve and to exclude alternative diagnosis such as pulmonary neoplasm seems to be a practically useful approach [29]. If the clinical probability of PTB is low, then early FOB may be carried out in such patients.
Limitation
In the present study, culture for Mycobacterium tuberculosis was not done due to lack of lab facilities.
Conclusion
Even though both induced sputum and bronchial washings were valuable for the diagnosis of tuberculosis in patients with sputum smear negative for AFB, bronchoscopy is a semi-invasive procedure, expensive and not readily available everywhere; whereas sputum induction procedure is simple, relatively safe, cost-effective, and is widely available. Therefore, in a patient with suspected sputum smear negative pulmonary tuberculosis sputum induction should be the initial procedure of choice, reserving bronchoscopy for those non responders seems to be a practically useful approach.